
PDF Publication Title:
Text from PDF Page: 008
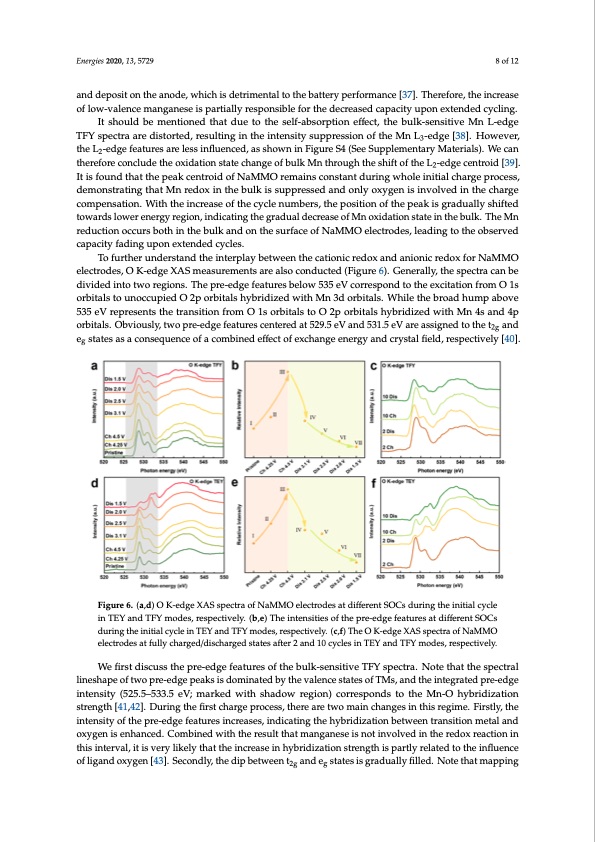
Energies 2020, 13, x FOR PEER REVIEW 8 of 12 Energies 2020, 13, 5729 8 of 12 the battery performance [37]. Therefore, the increase of low-valence manganese is partially responsible for the decreased capacity upon extended cycling. and deposit on the anode, which is detrimental to the battery performance [37]. Therefore, the increase It should be mentioned that due to the self-absorption effect, the bulk-sensitive Mn L-edge TFY of low-valence manganese is partially responsible for the decreased capacity upon extended cycling. spectra are distorted, resulting in the intensity suppression of the Mn L3-edge [38]. However, the L2- It should be mentioned that due to the self-absorption effect, the bulk-sensitive Mn L-edge edge features are less influenced, as shown in Figure S4 (See Supplementary Materials). We can TFY spectra are distorted, resulting in the intensity suppression of the Mn L3-edge [38]. However, therefore conclude the oxidation state change of bulk Mn through the shift of the L2-edge centroid the L2-edge features are less influenced, as shown in Figure S4 (See Supplementary Materials). We can [39]. It is found that the peak centroid of NaMMO remains constant during whole initial charge therefore conclude the oxidation state change of bulk Mn through the shift of the L2-edge centroid [39]. process, demonstrating that Mn redox in the bulk is suppressed and only oxygen is involved in the It is found that the peak centroid of NaMMO remains constant during whole initial charge process, charge compensation. With the increase of the cycle numbers, the position of the peak is gradually demonstrating that Mn redox in the bulk is suppressed and only oxygen is involved in the charge shifted towards lower energy region, indicating the gradual decrease of Mn oxidation state in the compensation. With the increase of the cycle numbers, the position of the peak is gradually shifted bulk. The Mn reduction occurs both in the bulk and on the surface of NaMMO electrodes, leading to towards lower energy region, indicating the gradual decrease of Mn oxidation state in the bulk. The Mn the observed capacity fading upon extended cycles. reduction occurs both in the bulk and on the surface of NaMMO electrodes, leading to the observed To further understand the interplay between the cationic redox and anionic redox for NaMMO capacity fading upon extended cycles. electrodes, O K-edge XAS measurements are also conducted (Figure 6). Generally, the spectra can be To further understand the interplay between the cationic redox and anionic redox for NaMMO divided into two regions. The pre-edge features below 535 eV correspond to the excitation from O 1s electrodes, O K-edge XAS measurements are also conducted (Figure 6). Generally, the spectra can be orbitals to unoccupied O 2p orbitals hybridized with Mn 3d orbitals. While the broad hump above divided into two regions. The pre-edge features below 535 eV correspond to the excitation from O 1s 535 eV represents the transition from O 1s orbitals to O 2p orbitals hybridized with Mn 4s and 4p orbitals to unoccupied O 2p orbitals hybridized with Mn 3d orbitals. While the broad hump above orbitals. Obviously, two pre-edge features centered at 529.5 eV and 531.5 eV are assigned to the t2g 535 eV represents the transition from O 1s orbitals to O 2p orbitals hybridized with Mn 4s and 4p and eg states as a consequence of a combined effect of exchange energy and crystal field, respectively orbitals. Obviously, two pre-edge features centered at 529.5 eV and 531.5 eV are assigned to the t2g and [40]. eg states as a consequence of a combined effect of exchange energy and crystal field, respectively [40]. Figure 6. (a,d) O K-edge XAS spectra of NaMMO electrodes at different SOCs during the initial cycle Figure 6. (a,d) O K-edge XAS spectra of NaMMO electrodes at different SOCs during the initial cycle in TEY and TFY modes, respectively. (b,e) The intensities of the pre-edge features at different SOCs in TEY and TFY modes, respectively. (b,e) The intensities of the pre-edge features at different SOCs during the initial cycle in TEY and TFY modes, respectively. (c,f) The O K-edge XAS spectra of NaMMO during the initial cycle in TEY and TFY modes, respectively. (c,f) The O K-edge XAS spectra of electrodes at fully charged/discharged states after 2 and 10 cycles in TEY and TFY modes, respectively. NaMMO electrodes at fully charged/discharged states after 2 and 10 cycles in TEY and TFY modes, lineshape of two pre-edge peaks is dominated by the valence states of TMs, and the integrated pre-edge respectively. We first discuss the pre-edge features of the bulk-sensitive TFY spectra. Note that the spectral We first discuss the pre-edge features of the bulk-sensitive TFY spectra. Note that the spectral intensity (525.5–533.5 eV; marked with shadow region) corresponds to the Mn-O hybridization lineshape of two pre-edge peaks is dominated by the valence states of TMs, and the integrated pre- strength [41,42]. During the first charge process, there are two main changes in this regime. Firstly, the edge intensity (525.5–533.5 eV; marked with shadow region) corresponds to the Mn-O hybridization intensity of the pre-edge features increases, indicating the hybridization between transition metal and strength [41,42]. During the first charge process, there are two main changes in this regime. Firstly, oxygen is enhanced. Combined with the result that manganese is not involved in the redox reaction in the intensity of the pre-edge features increases, indicating the hybridization between transition metal this interval, it is very likely that the increase in hybridization strength is partly related to the influence and oxygen is enhanced. Combined with the result that manganese is not involved in the redox of ligand oxygen [43]. Secondly, the dip between t2g and eg states is gradually filled. Note that mapping reaction in this interval, it is very likely that the increase in hybridization strength is partly related toPDF Image | Cathode Materials for Advanced Sodium-Ion Batteries
PDF Search Title:
Cathode Materials for Advanced Sodium-Ion BatteriesOriginal File Name Searched:
charge-compensation-sodium-ion-battery.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |