
PDF Publication Title:
Text from PDF Page: 003
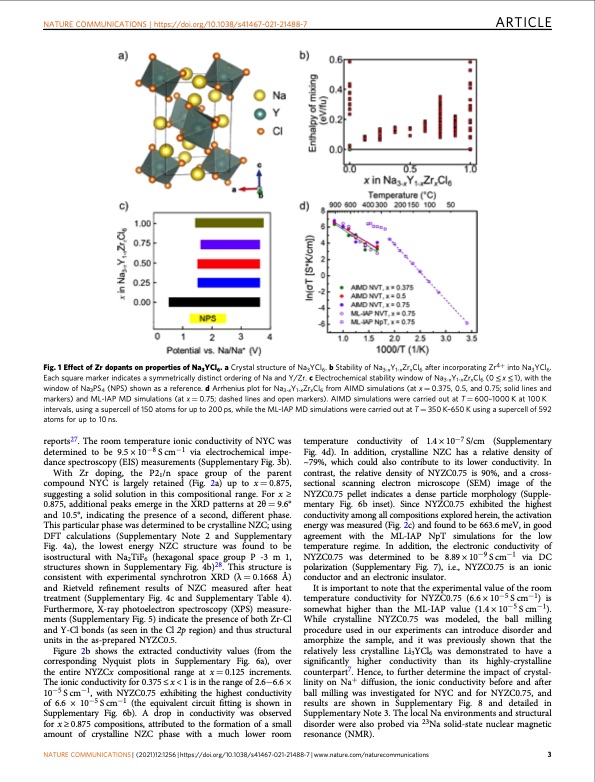
NATURE COMMUNICATIONS | https://doi.org/10.1038/s41467-021-21488-7 ARTICLE Fig. 1 Effect of Zr dopants on properties of Na3YCl6. a Crystal structure of Na3YCl6. b Stability of Na3-xY1-xZrxCl6 after incorporating Zr4+ into Na3YCl6. Each square marker indicates a symmetrically distinct ordering of Na and Y/Zr. c Electrochemical stability window of Na3-xY1-xZrxCl6 (0 ≤ x ≤ 1), with the window of Na3PS4 (NPS) shown as a reference. d Arrhenius plot for Na3-xY1-xZrxCl6 from AIMD simulations (at x = 0.375, 0.5, and 0.75; solid lines and markers) and ML-IAP MD simulations (at x = 0.75; dashed lines and open markers). AIMD simulations were carried out at T = 600–1000 K at 100 K intervals, using a supercell of 150 atoms for up to 200 ps, while the ML-IAP MD simulations were carried out at T = 350 K–650 K using a supercell of 592 atoms for up to 10 ns. reports27. The room temperature ionic conductivity of NYC was determined to be 9.5 × 10−8 S cm−1 via electrochemical impe- dance spectroscopy (EIS) measurements (Supplementary Fig. 3b). With Zr doping, the P21/n space group of the parent compound NYC is largely retained (Fig. 2a) up to x=0.875, suggesting a solid solution in this compositional range. For x ≥ 0.875, additional peaks emerge in the XRD patterns at 2θ = 9.6° and 10.5°, indicating the presence of a second, different phase. This particular phase was determined to be crystalline NZC; using DFT calculations (Supplementary Note 2 and Supplementary Fig. 4a), the lowest energy NZC structure was found to be isostructural with Na2TiF6 (hexagonal space group P -3 m 1, structures shown in Supplementary Fig. 4b)28. This structure is consistent with experimental synchrotron XRD (λ = 0.1668 Å) and Rietveld refinement results of NZC measured after heat treatment (Supplementary Fig. 4c and Supplementary Table 4). Furthermore, X-ray photoelectron spectroscopy (XPS) measure- ments (Supplementary Fig. 5) indicate the presence of both Zr-Cl and Y-Cl bonds (as seen in the Cl 2p region) and thus structural units in the as-prepared NYZC0.5. Figure 2b shows the extracted conductivity values (from the corresponding Nyquist plots in Supplementary Fig. 6a), over the entire NYZCx compositional range at x = 0.125 increments. The ionic conductivity for 0.375 ≤ x < 1 is in the range of 2.6−6.6 × 10−5 S cm−1, with NYZC0.75 exhibiting the highest conductivity of 6.6 × 10−5 S cm−1 (the equivalent circuit fitting is shown in Supplementary Fig. 6b). A drop in conductivity was observed for x ≥ 0.875 compositions, attributed to the formation of a small amount of crystalline NZC phase with a much lower room temperature conductivity of 1.4 × 10−7 S/cm (Supplementary Fig. 4d). In addition, crystalline NZC has a relative density of ~79%, which could also contribute to its lower conductivity. In contrast, the relative density of NYZC0.75 is 90%, and a cross- sectional scanning electron microscope (SEM) image of the NYZC0.75 pellet indicates a dense particle morphology (Supple- mentary Fig. 6b inset). Since NYZC0.75 exhibited the highest conductivity among all compositions explored herein, the activation energy was measured (Fig. 2c) and found to be 663.6 meV, in good agreement with the ML-IAP NpT simulations for the low temperature regime. In addition, the electronic conductivity of NYZC0.75 was determined to be 8.89 × 10−9 S cm−1 via DC polarization (Supplementary Fig. 7), i.e., NYZC0.75 is an ionic conductor and an electronic insulator. It is important to note that the experimental value of the room temperature conductivity for NYZC0.75 (6.6 × 10−5 S cm−1) is somewhat higher than the ML-IAP value (1.4 × 10−5 S cm−1). While crystalline NYZC0.75 was modeled, the ball milling procedure used in our experiments can introduce disorder and amorphize the sample, and it was previously shown that the relatively less crystalline Li3YCl6 was demonstrated to have a significantly higher conductivity than its highly-crystalline counterpart7. Hence, to further determine the impact of crystal- linity on Na+ diffusion, the ionic conductivity before and after ball milling was investigated for NYC and for NYZC0.75, and results are shown in Supplementary Fig. 8 and detailed in Supplementary Note 3. The local Na environments and structural disorder were also probed via 23Na solid-state nuclear magnetic resonance (NMR). NATURE COMMUNICATIONS | (2021)12:1256 | https://doi.org/10.1038/s41467-021-21488-7 | www.nature.com/naturecommunications 3PDF Image | cathode-solid electrolyte composite sodium-ion
PDF Search Title:
cathode-solid electrolyte composite sodium-ionOriginal File Name Searched:
s41467-021-21488-7.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |