
PDF Publication Title:
Text from PDF Page: 005
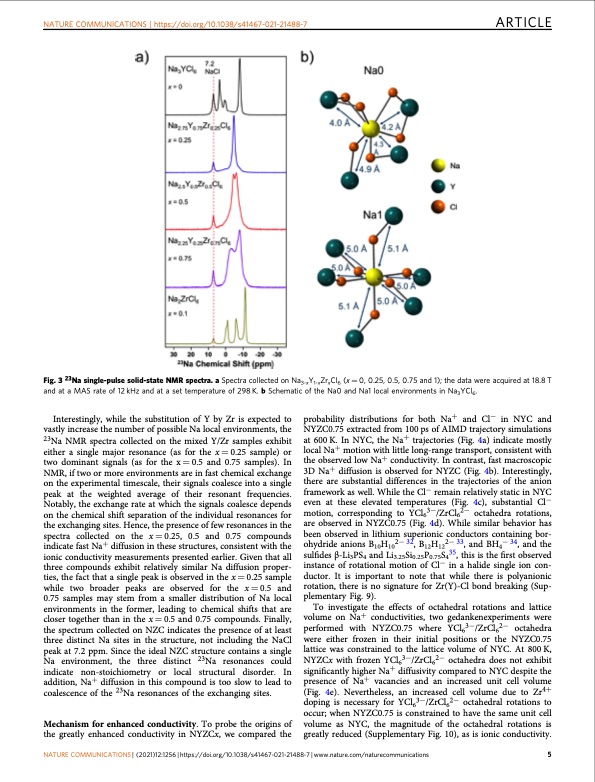
NATURE COMMUNICATIONS | https://doi.org/10.1038/s41467-021-21488-7 ARTICLE Fig. 3 23Na single-pulse solid-state NMR spectra. a Spectra collected on Na3-xY1-xZrxCl6 (x = 0, 0.25, 0.5, 0.75 and 1); the data were acquired at 18.8 T and at a MAS rate of 12 kHz and at a set temperature of 298 K. b Schematic of the Na0 and Na1 local environments in Na3YCl6. Interestingly, while the substitution of Y by Zr is expected to vastly increase the number of possible Na local environments, the 23Na NMR spectra collected on the mixed Y/Zr samples exhibit either a single major resonance (as for the x = 0.25 sample) or two dominant signals (as for the x = 0.5 and 0.75 samples). In NMR, if two or more environments are in fast chemical exchange on the experimental timescale, their signals coalesce into a single peak at the weighted average of their resonant frequencies. Notably, the exchange rate at which the signals coalesce depends on the chemical shift separation of the individual resonances for the exchanging sites. Hence, the presence of few resonances in the spectra collected on the x = 0.25, 0.5 and 0.75 compounds indicate fast Na+ diffusion in these structures, consistent with the ionic conductivity measurements presented earlier. Given that all three compounds exhibit relatively similar Na diffusion proper- ties, the fact that a single peak is observed in the x = 0.25 sample while two broader peaks are observed for the x = 0.5 and 0.75 samples may stem from a smaller distribution of Na local environments in the former, leading to chemical shifts that are closer together than in the x = 0.5 and 0.75 compounds. Finally, the spectrum collected on NZC indicates the presence of at least three distinct Na sites in the structure, not including the NaCl peak at 7.2 ppm. Since the ideal NZC structure contains a single Na environment, the three distinct 23Na resonances could indicate non-stoichiometry or local structural disorder. In addition, Na+ diffusion in this compound is too slow to lead to coalescence of the 23Na resonances of the exchanging sites. Mechanism for enhanced conductivity. To probe the origins of the greatly enhanced conductivity in NYZCx, we compared the probability distributions for both Na+ and Cl− in NYC and NYZC0.75 extracted from 100 ps of AIMD trajectory simulations at 600 K. In NYC, the Na+ trajectories (Fig. 4a) indicate mostly local Na+ motion with little long-range transport, consistent with the observed low Na+ conductivity. In contrast, fast macroscopic 3D Na+ diffusion is observed for NYZC (Fig. 4b). Interestingly, there are substantial differences in the trajectories of the anion framework as well. While the Cl− remain relatively static in NYC even at these elevated temperatures (Fig. 4c), substantial Cl− motion, corresponding to YCl63−/ZrCl62− octahedra rotations, are observed in NYZC0.75 (Fig. 4d). While similar behavior has been observed in lithium superionic conductors containing bor- ohydride anions B10H102− 32, B12H122− 33, and BH4− 34, and the sulfides β-Li3PS4 and Li3.25Si0.25P0.75S435, this is the first observed instance of rotational motion of Cl− in a halide single ion con- ductor. It is important to note that while there is polyanionic rotation, there is no signature for Zr(Y)-Cl bond breaking (Sup- plementary Fig. 9). To investigate the effects of octahedral rotations and lattice volume on Na+ conductivities, two gedankenexperiments were performed with NYZC0.75 where YCl63−/ZrCl62− octahedra were either frozen in their initial positions or the NYZC0.75 lattice was constrained to the lattice volume of NYC. At 800 K, NYZCx with frozen YCl63−/ZrCl62− octahedra does not exhibit significantly higher Na+ diffusivity compared to NYC despite the presence of Na+ vacancies and an increased unit cell volume (Fig. 4e). Nevertheless, an increased cell volume due to Zr4+ doping is necessary for YCl63−/ZrCl62− octahedral rotations to occur; when NYZC0.75 is constrained to have the same unit cell volume as NYC, the magnitude of the octahedral rotations is greatly reduced (Supplementary Fig. 10), as is ionic conductivity. NATURE COMMUNICATIONS | (2021)12:1256 | https://doi.org/10.1038/s41467-021-21488-7 | www.nature.com/naturecommunications 5PDF Image | cathode-solid electrolyte composite sodium-ion
PDF Search Title:
cathode-solid electrolyte composite sodium-ionOriginal File Name Searched:
s41467-021-21488-7.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |