
PDF Publication Title:
Text from PDF Page: 008
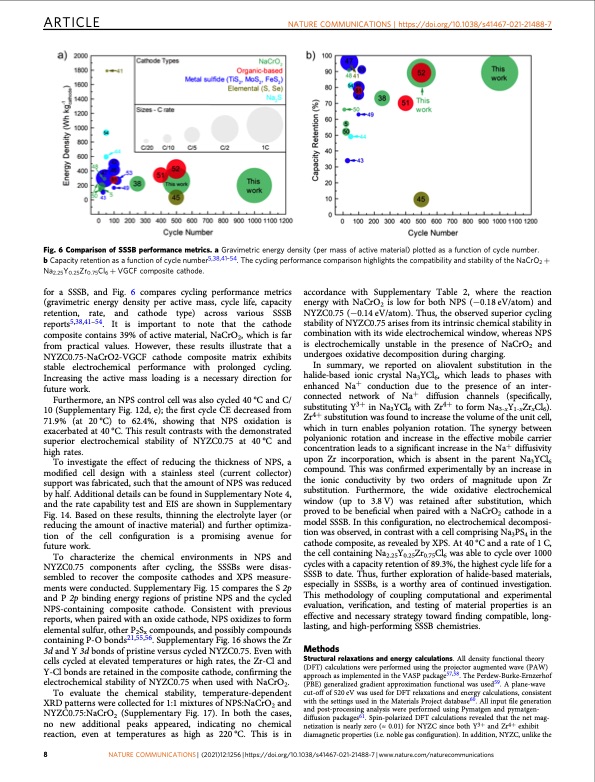
ARTICLE NATURE COMMUNICATIONS | https://doi.org/10.1038/s41467-021-21488-7 Fig. 6 Comparison of SSSB performance metrics. a Gravimetric energy density (per mass of active material) plotted as a function of cycle number. b Capacity retention as a function of cycle number5,38,41–54. The cycling performance comparison highlights the compatibility and stability of the NaCrO2 + Na2.25Y0.25Zr0.75Cl6 + VGCF composite cathode. for a SSSB, and Fig. 6 compares cycling performance metrics (gravimetric energy density per active mass, cycle life, capacity retention, rate, and cathode type) across various SSSB reports5,38,41–54. It is important to note that the cathode composite contains 39% of active material, NaCrO2, which is far from practical values. However, these results illustrate that a NYZC0.75-NaCrO2-VGCF cathode composite matrix exhibits stable electrochemical performance with prolonged cycling. Increasing the active mass loading is a necessary direction for future work. Furthermore, an NPS control cell was also cycled 40 °C and C/ 10 (Supplementary Fig. 12d, e); the first cycle CE decreased from 71.9% (at 20°C) to 62.4%, showing that NPS oxidation is exacerbated at 40 °C. This result contrasts with the demonstrated superior electrochemical stability of NYZC0.75 at 40°C and high rates. To investigate the effect of reducing the thickness of NPS, a modified cell design with a stainless steel (current collector) support was fabricated, such that the amount of NPS was reduced by half. Additional details can be found in Supplementary Note 4, and the rate capability test and EIS are shown in Supplementary Fig. 14. Based on these results, thinning the electrolyte layer (or reducing the amount of inactive material) and further optimiza- tion of the cell configuration is a promising avenue for future work. To characterize the chemical environments in NPS and NYZC0.75 components after cycling, the SSSBs were disas- sembled to recover the composite cathodes and XPS measure- ments were conducted. Supplementary Fig. 15 compares the S 2p and P 2p binding energy regions of pristine NPS and the cycled NPS-containing composite cathode. Consistent with previous reports, when paired with an oxide cathode, NPS oxidizes to form elemental sulfur, other P2Sx compounds, and possibly compounds containing P-O bonds21,55,56. Supplementary Fig. 16 shows the Zr 3d and Y 3d bonds of pristine versus cycled NYZC0.75. Even with cells cycled at elevated temperatures or high rates, the Zr-Cl and Y-Cl bonds are retained in the composite cathode, confirming the electrochemical stability of NYZC0.75 when used with NaCrO2. accordance with Supplementary Table 2, where the reaction energy with NaCrO2 is low for both NPS (−0.18 eV/atom) and NYZC0.75 (−0.14 eV/atom). Thus, the observed superior cycling stability of NYZC0.75 arises from its intrinsic chemical stability in combination with its wide electrochemical window, whereas NPS is electrochemically unstable in the presence of NaCrO2 and undergoes oxidative decomposition during charging. In summary, we reported on aliovalent substitution in the halide-based ionic crystal Na3YCl6, which leads to phases with enhanced Na+ conduction due to the presence of an inter- connected network of Na+ diffusion channels (specifically, substituting Y3+ in Na3YCl6 with Zr4+ to form Na3-xY1-xZrxCl6). Zr4+ substitution was found to increase the volume of the unit cell, which in turn enables polyanion rotation. The synergy between polyanionic rotation and increase in the effective mobile carrier concentration leads to a significant increase in the Na+ diffusivity upon Zr incorporation, which is absent in the parent Na3YCl6 compound. This was confirmed experimentally by an increase in the ionic conductivity by two orders of magnitude upon Zr substitution. Furthermore, the wide oxidative electrochemical window (up to 3.8V) was retained after substitution, which proved to be beneficial when paired with a NaCrO2 cathode in a model SSSB. In this configuration, no electrochemical decomposi- tion was observed, in contrast with a cell comprising Na3PS4 in the cathode composite, as revealed by XPS. At 40 °C and a rate of 1 C, the cell containing Na2.25Y0.25Zr0.75Cl6 was able to cycle over 1000 cycles with a capacity retention of 89.3%, the highest cycle life for a SSSB to date. Thus, further exploration of halide-based materials, especially in SSSBs, is a worthy area of continued investigation. This methodology of coupling computational and experimental evaluation, verification, and testing of material properties is an effective and necessary strategy toward finding compatible, long- lasting, and high-performing SSSB chemistries. Methods Structural relaxations and energy calculations. All density functional theory (DFT) calculations were performed using the projector augmented wave (PAW) approach as implemented in the VASP package57,58. The Perdew-Burke-Ernzerhof (PBE) generalized gradient approximation functional was used59. A plane-wave cut-off of 520 eV was used for DFT relaxations and energy calculations, consistent with the settings used in the Materials Project database60. All input file generation and post-processing analysis were performed using Pymatgen and pymatgen- diffusion packages61. Spin-polarized DFT calculations revealed that the net mag- netization is nearly zero (≈ 0.01) for NYZC since both Y3+ and Zr4+ exhibit diamagnetic properties (i.e. noble gas configuration). In addition, NYZC, unlike the To evaluate the chemical stability, temperature-dependent XRD patterns were collected for 1:1 mixtures of NPS:NaCrO2 and NYZC0.75:NaCrO (Supplementary Fig. 17). In both the cases, 2 no new additional peaks appeared, indicating no chemical reaction, even at temperatures as high as 220°C. This is in 8 NATURE COMMUNICATIONS | (2021)12:1256 | https://doi.org/10.1038/s41467-021-21488-7 | www.nature.com/naturecommunicationsPDF Image | cathode-solid electrolyte composite sodium-ion
PDF Search Title:
cathode-solid electrolyte composite sodium-ionOriginal File Name Searched:
s41467-021-21488-7.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |