
PDF Publication Title:
Text from PDF Page: 008
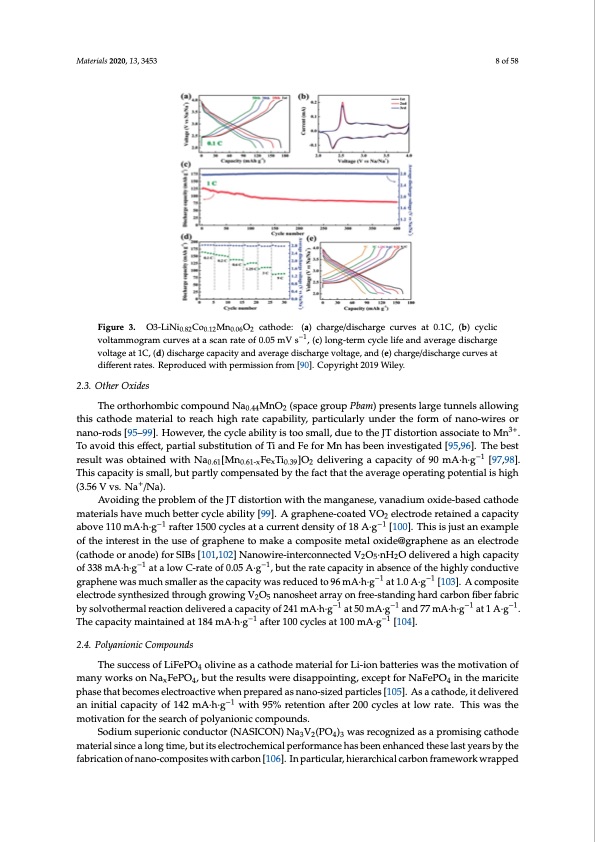
voltage, and Mn plays a role in stabilizing the structure during Na+ extraction/insertion. Indeed, the charge/discharge curves in Figure 3a are very smooth, thus avoiding the voltage plateaus associated to the gliding of transition metal oxide slabs and Na+/vacancy ordering. A pair of sharp redox peaks at 2.5 V (oxidation) and 2.3 V (reduction) in Figure 3b reveals the phase transition between O3 and PM3a.terTiahlse20h20i,g1h3l,y345c3oincident curves in the initial three cycles for Na-NCM811 indicate a g8oof5d8 reversibility of phase transition. Figure 3. O3-LiNi0.82 Co0.12 Mn0.06 O2 cathode: (a) charge/discharge curves at 0.1C, (b) cyclic Figure 3. O3-LiNi0.82Co0.12Mn0.06O2 cathode: (a) charge/discharge curves at 0.1C, (b) cyclic voltammogram curves at a scan rate of 0.05 mV s−1, (c) long-term cycle life and average discharge voltammogram curves at a scan rate of 0.05 mV s−1, (c) long-term cycle life and average discharge voltage at 1C, (d) discharge capacity and average discharge voltage, and (e) charge/discharge curves at voltage at 1C, (d) discharge capacity and average discharge voltage, and (e) charge/discharge curves different rates. Reproduced with permission from [90]. Copyright 2019 Wiley. at different rates. Reproduced with permission from [90]. Copyright 2019 Wiley. 2.3. Other Oxides The orthorhombic compound Na0.44MnO2 (space group Pbam) presents large tunnels allowing this cathode material to reach high rate capability, particularly under the form of nano-wires or nano-rods [95–99]. However, the cycle ability is too small, due to the JT distortion associate to Mn3+. To avoid this effect, partial substitution of Ti and Fe for Mn has been investigated [95,96]. The best result was obtained with Na0.61[Mn0.61-xFexTi0.39]O2 delivering a capacity of 90 mA·h·g−1 [97,98]. This capacity is small, but partly compensated by the fact that the average operating potential is high (3.56 V vs. Na+/Na). Avoiding the problem of the JT distortion with the manganese, vanadium oxide-based cathode materials have much better cycle ability [99]. A graphene-coated VO2 electrode retained a capacity above 110 mA·h·g−1 rafter 1500 cycles at a current density of 18 A·g−1 [100]. This is just an example of the interest in the use of graphene to make a composite metal oxide@graphene as an electrode (cathode or anode) for SIBs [101,102] Nanowire-interconnected V2O5·nH2O delivered a high capacity of 338 mA·h·g−1 at a low C-rate of 0.05 A·g−1, but the rate capacity in absence of the highly conductive graphene was much smaller as the capacity was reduced to 96 mA·h·g−1 at 1.0 A·g−1 [103]. A composite electrode synthesized through growing V2O5 nanosheet array on free-standing hard carbon fiber fabric by solvothermal reaction delivered a capacity of 241 mA·h·g−1 at 50 mA·g−1 and 77 mA·h·g−1 at 1 A·g−1. The capacity maintained at 184 mA·h·g−1 after 100 cycles at 100 mA·g−1 [104]. 2.4. Polyanionic Compounds The success of LiFePO4 olivine as a cathode material for Li-ion batteries was the motivation of many works on NaxFePO4, but the results were disappointing, except for NaFePO4 in the maricite phase that becomes electroactive when prepared as nano-sized particles [105]. As a cathode, it delivered an initial capacity of 142 mA·h·g−1 with 95% retention after 200 cycles at low rate. This was the motivation for the search of polyanionic compounds. Sodium superionic conductor (NASICON) Na3V2(PO4)3 was recognized as a promising cathode material since a long time, but its electrochemical performance has been enhanced these last years by the fabrication of nano-composites with carbon [106]. In particular, hierarchical carbon framework wrappedPDF Image | Electrode Materials for Sodium-Ion Batteries
PDF Search Title:
Electrode Materials for Sodium-Ion BatteriesOriginal File Name Searched:
materials-13-03453-v2.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |