
PDF Publication Title:
Text from PDF Page: 030
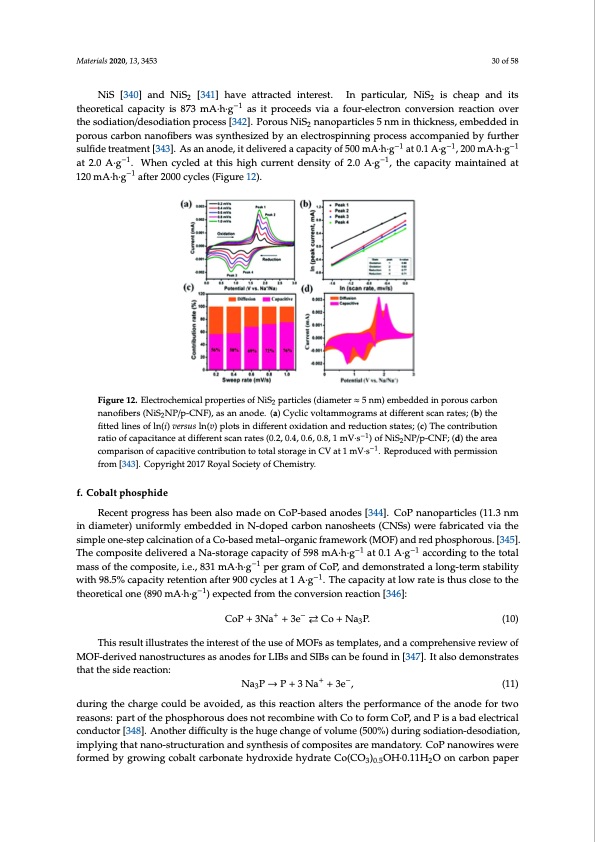
Materials 2020, 13, 3453 30 of 58 NiS [340] and NiS2 [341] have attracted interest. In particular, NiS2 is cheap and its theoretical capacity is 873 mA·h·g−1 as it proceeds via a four-electron conversion reaction over the sodiation/desodiation process [342]. Porous NiS2 nanoparticles 5 nm in thickness, embedded in porous carbon nanofibers was synthesized by an electrospinning process accompanied by further sulfide treatment [343]. As an anode, it delivered a capacity of 500 mA·h·g−1 at 0.1 A·g−1, 200 mA·h·g−1 at 2.0 A·g−1. When cycled at this high current density of 2.0 A·g−1, the capacity maintained at Materials 2020, 13, x FOR PEER REVIEW 28 of 53 120 mA·h·g−1 after 2000 cycles (Figure 12). Figure 12. Electrochemical properties of NiS2 particles (diameter ≈ 5 nm) embedded in porous carbon Figure 12. Electrochemical properties of NiS2 particles (diameter ≈ 5 nm) embedded in porous carbon nanofibers (NiS2NP/p-CNF), as an anode. (a) Cyclic voltammograms at different scan rates; (b) the nanofibers (NiS2NP/p-CNF), as an anode. (a) Cyclic voltammograms at different scan rates; (b) the fitted lines of ln(i) versus ln(v) plots in different oxidation and reduction states; (c) The contribution fitted lines of ln(i) versus ln(v) plots in different oxidation and reduction states; (c) The contribution ratio of capacitance at different scan rates (0.2, 0.4, 0.6, 0.8, 1 mV·s−1) of NiS2NP/p-CNF; (d) the area ratio of capacitance at different scan rates (0.2, 0.4, 0.6, 0.8, 1 mV s−1) of NiS2NP/p-CNF; (d) the area comparison of capacitive contribution to total storage in CV at 1 mV·s−1. Reproduced with permission comparison of capacitive contribution to total storage in CV at 1 mV s−1. Reproduced with permission from [343]. Copyright 2017 Royal Society of Chemistry. from [343]. Copyright 2017 Royal Society of Chemistry. f. Cobalt phosphide f. Cobalt phosphide Recent progress has been also made on CoP-based anodes [344]. CoP nanoparticles (11.3 nm Recent progress has been also made on CoP-based anodes [344]. CoP nanoparticles (11.3 nm in in diameter) uniformly embedded in N-doped carbon nanosheets (CNSs) were fabricated via the diameter) uniformly embedded in N-doped carbon nanosheets (CNSs) were fabricated via the simple simple one-step calcination of a Co-based metal–organic framework (MOF) and red phosphorous. [345]. one-step calcination of a Co-based metal–organic framework (MOF) and red phosphorous. [345]. The The composite delivered a Na-storage capacity of 598 mA·h·g−1 at 0.1 A·g−1 according to the total composite delivered a Na-storage capacity of 598 mAh∙g−1 at 0.1 A∙g−1 according to the total mass of mass of the composite, i.e., 831 mA·h·g−1 per gram of CoP, and demonstrated a long-term stability the composite, i.e., 831 mAh∙g−1 per gram of CoP, and demonstrated a long-term stability with 98.5% with 98.5% capacity retention after 900 cycles at 1 A·g−1. The capacity at low rate is thus close to the capacity retention after 900 cycles at 1 A∙g−1. The capacity at low rate is thus close to the theoretical theoretical one (890 mA·h·g−1) expected from the conversion reaction [346]: one (890 mAh g−1) expected from the conversion reaction [346]: ++−− CoP+3Na +3e ⇄Co+Na3P. (10) CoP+3Na +3e Co+Na3P. (10) This result illustrates the interest of the use of MOFs as templates, and a comprehensive review This result illustrates the interest of the use of MOFs as templates, and a comprehensive review of of MOF-derived nanostructures as anodes for LIBs and SIBs can be found in [347]. It also MOF-derived nanostructures as anodes for LIBs and SIBs can be found in [347]. It also demonstrates demonstrates that the side reaction: that the side reaction: ++−− NaNPa→3P P→+P3+N3aNa+ +3e3e, , ((11)) 3 during the charge could be avoided, as this reaction alters the performance of the anode for two during the charge could be avoided, as this reaction alters the performance of the anode for two reasons: part of the phosphorous does not recombine with Co to form CoP, and P is a bad electrical reasons: part of the phosphorous does not recombine with Co to form CoP, and P is a bad electrical conductor [348]. Another difficulty is the huge change of volume (500%) during sodiation- conductor [348]. Another difficulty is the huge change of volume (500%) during sodiation-desodiation, desodiation, implying that nano-structuration and synthesis of composites are mandatory. CoP implying that nano-structuration and synthesis of composites are mandatory. CoP nanowires were nanowires were formed by growing cobalt carbonate hydroxide hydrate Co(CO3)0.5OH∙0.11H2O on formed by growing cobalt carbonate hydroxide hydrate Co(CO3)0.5OH·0.11H2O on carbon paper carbon paper substrate. Then, the CoP wires were coated with polypyrrole by a simple in-situ polymerization process [349]. The 5-nm thick polypyrrole coating layer was able to buffer the change of volume of the CoP nanowires (50 nm in diameter), and the carbon paper was used as a conductor. Consequently, the anode delivered an areal capacity of 0.443 mAh cm−2 at 1.5 mA cm−2 after the first cycles, without capacity fading over 1000 cycles. At the high current density of 3 mA cm−2, the discharge capacity of 0.285 mAh cm−2 was maintained. Like in the case of the other cobalt compoundsPDF Image | Electrode Materials for Sodium-Ion Batteries
PDF Search Title:
Electrode Materials for Sodium-Ion BatteriesOriginal File Name Searched:
materials-13-03453-v2.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |