
PDF Publication Title:
Text from PDF Page: 010
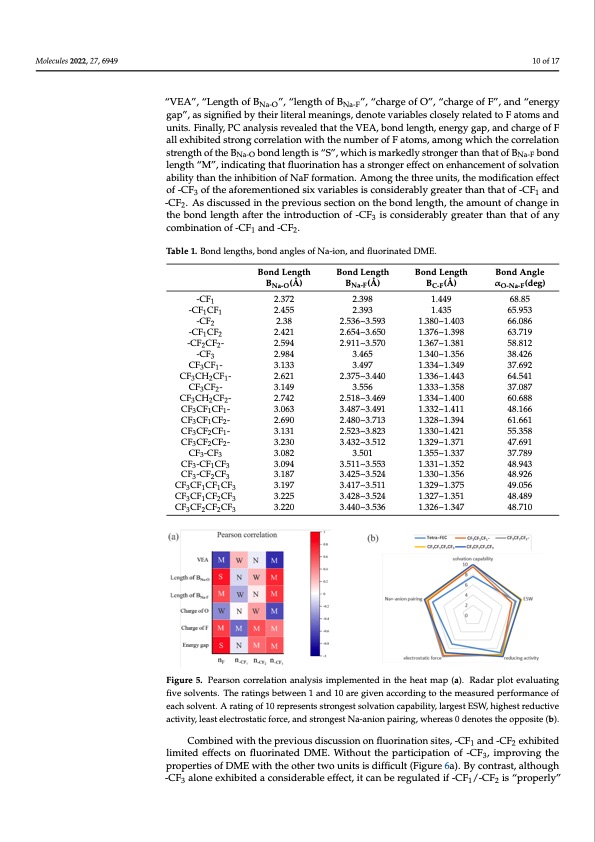
Molecules 2022, 27, x FOR PEER REVIEW 10 of 17 Molecules 2022, 27, 6949 CF3CF2CF2- CF3-CF3 CF3-CF1CF3 3.230 3.082 3.432~3.512 3.501 Na-F 1.329~1.371 1.355~1.337 47.691 37.78910 of 17 48.943 48.926 CF -CF CF 323 3.094 3.187 3.511~3.553 3.425~3.524 1.331~1.352 1.330~1.356 “VEA”, “Length of B gap”C, aFsCsiFgnCiFfieCdFby their literal meanings, denote variables closely related to F atoms and 3113 3.197 3.417~3.511 1.329~1.375 49.056 CF CF CF CF 3123 Na-O ”, “length of B ”, “charge of O”, “charge of F”, and “energy units. Finally, PC analysis revealed that the VEA, bond length, energy gap, and charge of F 3.225 3.428~3.524 1.327~1.351 48.489 all exhibited strong correlation with the number of F atoms, among which the correlation strenCgtFhCoFf tCheFBCF bond length is “S”, which is markedly stronger than that of B bond Na-O 3.220 3.440~3.536 1.326~1.347 48.N7a1-0F 3223 length “M”, indicating that fluorination has a stronger effect on enhancement of solvation ability than the inhibition of NaF formation. Among the three units, the modification effect Because of the complexity of the problem, Pearson correlation (PC) analysis [46] was of -CF3 of the aforementioned six variables is considerably greater than that of -CF1 and used to understand the close relationship among properties and the types, numbers, and -CF2. As discussed in the previous section on the bond length, the amount of change in positions of the three units. The Pearson correction coefficient is between −1 and 1, with −1 the bond length after the introduction of -CF3 is considerably greater than that of any indicating a perfectly negative linear correlation, 1 indicating a perfectly positive linear cor- combination of -CF1 and -CF2. relation, and 0 indicating no linear relationship at all. The Pearson correction coefficient is calculated as follows: Table 1. Bond lengths, bond angles of Na-ion, and fluorinated DME. ∑𝑛 (𝑥 −𝑥̅)(𝑦 −𝑦̅) -CF1 𝑃𝐶= 𝑖=1𝑖 𝑖 , (3) Bond Length Bond Length Bond Length Bond Angle B 𝑛2𝑛2 (Å)√∑ (𝑥B−𝑥(Å̅))√∑ (𝑦B−𝑦̅(Å)) α (deg) 68.85 𝑥𝑦 Na-O 𝑖=1 𝑖Na-F 𝑖=1 𝑖 C-F 2.372 2.398 1.449 O-Na-F where x and y denote two random variables, i denotes the index in the regression sample, and -CF1 CF1 2.455 2.393 1.435 65.953 n denotes the number of samples. In this study, we define four correlation strengths “strong”, -CF2 2.38 2.536~3.593 1.380~1.403 66.086 “medium”, “weak”, and “negligible”, corresponding to the cases when the absolute values of -CF1 CF2 2.421 2.654~3.650 1.376~1.398 63.719 thePC-CcFoeCffFici-entsfallinth2.e59in4tervals(0.82,.19.101)~,(30.5.47,00.8),(0.21,.306.47)~,1a.n38d1(0.0,0.2),r5e8sp.8e1c2tively 22 (abbrevi-aCteFd3 asS,M,W,a2n.d98N4,respectively3).t4o65improverea1d.3a4b0i~li1t.y3.56Figure5ad3is8p.4l2a6ysthe CF CF - 3.133 3.497 1.334~1.349 37.692 PC coeffi3cien1ts of several variables in the heat map, with the labels “nF”, “n-CF1 ”, “n-CF2 ”, and CF3 CH2 CF1 - 2.621 2.375~3.440 1.336~1.443 64.541 “n-CF3 ” denoting the number of F atoms in each of the three units. “VEA”, “Length of BNa-O”, CF3 CF2 - 3.149 3.556 1.333~1.358 37.087 “length of BNa-F”, “charge of O”, “charge of F”, and “energy gap”, as signified by their literal CF3 CH2 CF2 - 2.742 2.518~3.469 1.334~1.400 60.688 meanings, denote variables closely related to F atoms and units. Finally, PC analysis revealed CF3 CF1 CF1 - 3.063 3.487~3.491 1.332~1.411 48.166 thatCthFeCVFEAC,Fb-ondlength2,.e6n9e0rgygap,an2d.48ch0~a3r.g7e13ofFallex1.h3i2b8i~te1d.39s4trongcorre6la1t.6io6n1with 312 CF CF CF - 3.131 2.523~3.823 1.330~1.421 55.358 the num3 be2r of1F atoms, among which the correlation strength of the BNa-O bond length is “S”, CF3 CF2 CF2 - 3.230 3.432~3.512 1.329~1.371 47.691 which is markedly stronger than that of BNa-F bond length “M”, indicating that fluorination CF3 -CF3 3.082 3.501 1.355~1.337 37.789 has a stronger effect on enhancement of solvation ability than the inhibition of NaF for- CF3 -CF1 CF3 3.094 3.511~3.553 1.331~1.352 48.943 mation. Among the three units, the modification effect of -CF3 of the aforementioned six CF3 -CF2 CF3 3.187 3.425~3.524 1.330~1.356 48.926 variables is considerably greater than that of -CF1 and -CF2. As discussed in the previous CF3 CF1 CF1 CF3 3.197 3.417~3.511 1.329~1.375 49.056 secCtioF3nCoFn1CthF2eCbFo3nd length3,.2t2h5e amount o3f.c4h28a~n3g.e52i4n the bon1.d32l7e~n1g.t3h51after the in4t8ro.4d8u9ction CF CF CF CF 3.220 3.440~3.536 1.326~1.347 48.710 of -CF33 is2con2sid3erably greater than that of any combination of -CF1 and -CF2. FFigiguurere5.5.PePaerasorsnocnocrroerlraetliaotnioannalnyasliyssiimspimlepmlemntendteindtihnetheathmeaatpm(ap).R(a)d.aRrapdloatrepvlaoltueavtianlguafitvineg solvents. The ratings between 1 and 10 are given according to the measured performance of each five solvents. The ratings between 1 and 10 are given according to the measured performance of solvent. A rating of 10 represents strongest solvation capability, largest ESW, highest reductive ac- each solvent. A rating of 10 represents strongest solvation capability, largest ESW, highest reductive tivity, least electrostatic force, and strongest Na-anion pairing, whereas 0 denotes the opposite (b). activity, least electrostatic force, and strongest Na-anion pairing, whereas 0 denotes the opposite (b). Combined with the previous discussion on fluorination sites, -CF1 and -CF2 exhibited limited effects on fluorinated DME. Without the participation of -CF3, improving the properties of DME with the other two units is difficult (Figure 6a). By contrast, although -CF3 alone exhibited a considerable effect, it can be regulated if -CF1/-CF2 is “properly”PDF Image | First-Principles-Based Optimized Design of Fluoride Electrolytes
PDF Search Title:
First-Principles-Based Optimized Design of Fluoride ElectrolytesOriginal File Name Searched:
molecules-27-06949.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |