
PDF Publication Title:
Text from PDF Page: 003
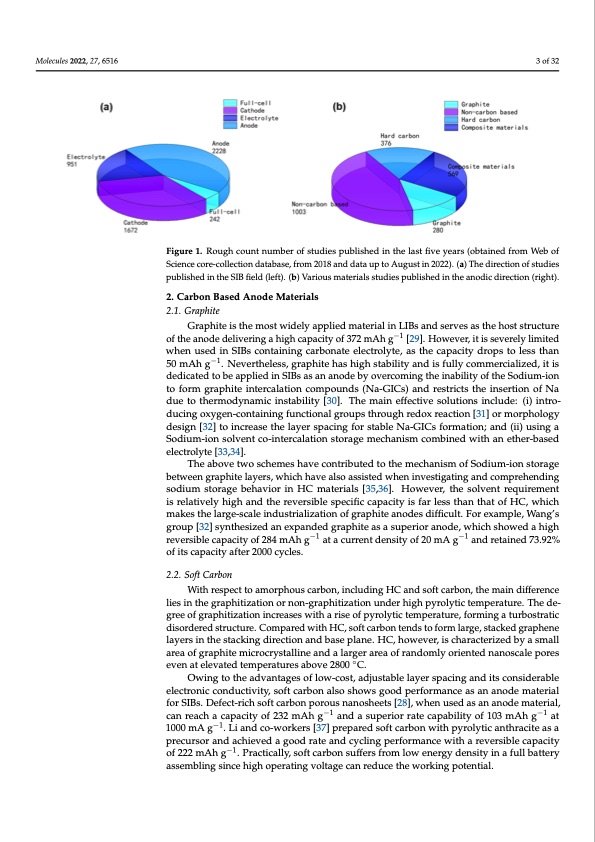
Molecules 2022, 27, 6516 sodium storage capacity at 300.6 mAh g−1 and the initial Coulombic efficiency (ICE) at 88.6% [26]. Herein, we summarize the different carbon-based anode materials used in SIBs, such as graphite, soft carbon, and hard carbon (HC), which is also called non-graphitization carbon. Moreover, this review summarizes the work and progress of predecessors, the sodium storage mechanism, and the modification and advantages of HC. 3 of 32 Figure 1. Rough count number of studies published in the last five years (obtained from Web of Science core-collection database, from 2018 and data up to August in 2022). (a) The direction of studies published in the SIB field (left). (b) Various materials studies published in the anodic direction (right). 2. Carbon Based Anode Materials 2.1. Graphite Graphite is the most widely applied material in LIBs and serves as the host structure of the anode delivering a high capacity of 372 mAh g−1 [29]. However, it is severely limited when used in SIBs containing carbonate electrolyte, as the capacity drops to less than 50 mAh g−1. Nevertheless, graphite has high stability and is fully commercialized, it is dedicated to be applied in SIBs as an anode by overcoming the inability of the Sodium-ion to form graphite intercalation compounds (Na-GICs) and restricts the insertion of Na due to thermodynamic instability [30]. The main effective solutions include: (i) intro- ducing oxygen-containing functional groups through redox reaction [31] or morphology design [32] to increase the layer spacing for stable Na-GICs formation; and (ii) using a Sodium-ion solvent co-intercalation storage mechanism combined with an ether-based electrolyte [33,34]. The above two schemes have contributed to the mechanism of Sodium-ion storage between graphite layers, which have also assisted when investigating and comprehending sodium storage behavior in HC materials [35,36]. However, the solvent requirement is relatively high and the reversible specific capacity is far less than that of HC, which makes the large-scale industrialization of graphite anodes difficult. For example, Wang’s group [32] synthesized an expanded graphite as a superior anode, which showed a high reversible capacity of 284 mAh g−1 at a current density of 20 mA g−1 and retained 73.92% of its capacity after 2000 cycles. 2.2. Soft Carbon With respect to amorphous carbon, including HC and soft carbon, the main difference lies in the graphitization or non-graphitization under high pyrolytic temperature. The de- gree of graphitization increases with a rise of pyrolytic temperature, forming a turbostratic disordered structure. Compared with HC, soft carbon tends to form large, stacked graphene layers in the stacking direction and base plane. HC, however, is characterized by a small area of graphite microcrystalline and a larger area of randomly oriented nanoscale pores even at elevated temperatures above 2800 ◦C. Owing to the advantages of low-cost, adjustable layer spacing and its considerable electronic conductivity, soft carbon also shows good performance as an anode material for SIBs. Defect-rich soft carbon porous nanosheets [28], when used as an anode material, can reach a capacity of 232 mAh g−1 and a superior rate capability of 103 mAh g−1 at 1000 mA g−1. Li and co-workers [37] prepared soft carbon with pyrolytic anthracite as a precursor and achieved a good rate and cycling performance with a reversible capacity of 222 mAh g−1. Practically, soft carbon suffers from low energy density in a full battery assembling since high operating voltage can reduce the working potential.PDF Image | Hard Carbons as Anodes in Sodium-Ion Batteries
PDF Search Title:
Hard Carbons as Anodes in Sodium-Ion BatteriesOriginal File Name Searched:
molecules-27-06516-v2.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |