
PDF Publication Title:
Text from PDF Page: 012
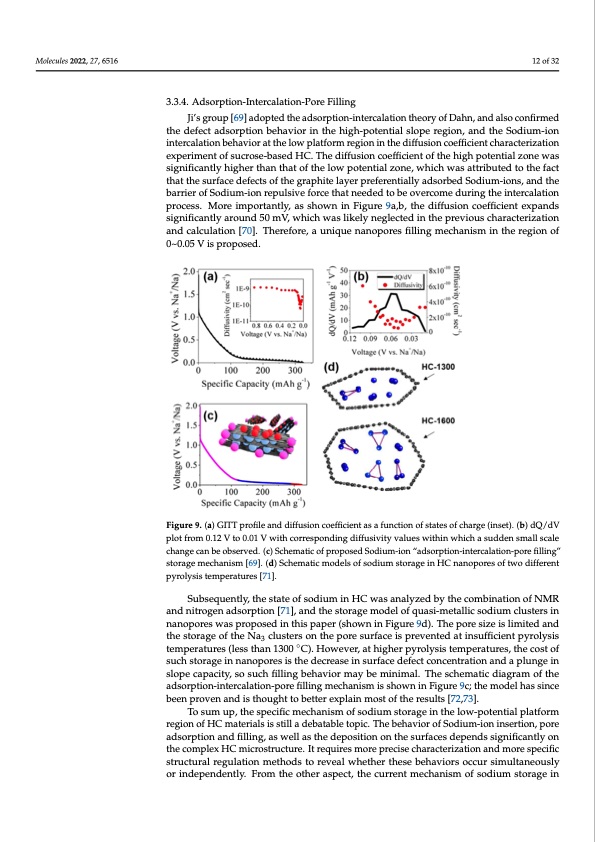
Molecules 2022, 27, x FOR PEER REVIEW 13 of 34 Molecules 2022, 27, 6516 12 of 32 troscopy, electrochemical characterization, and Density Functional Theory (DFT) simu- lation. 3.3.4. Adsorption-Intercalation-Pore Filling 3.3.4. Adsorption-Intercalation-Pore Filling Ji’s group [69] adopted the adsorption-intercalation theory of Dahn, and also confirmed Ji’s group [69] adopted the adsorption-intercalation theory of Dahn, and also con- the defect adsorption behavior in the high-potential slope region, and the Sodium-ion firmed the defect adsorption behavior in the high-potential slope region, and the Sodi- intercalation behavior at the low platform region in the diffusion coefficient characterization um-ion intercalation behavior at the low platform region in the diffusion coefficient experiment of sucrose-based HC. The diffusion coefficient of the high potential zone was characterization experiment of sucrose-based HC. The diffusion coefficient of the high significantly higher than that of the low potential zone, which was attributed to the fact potential zone was significantly higher than that of the low potential zone, which was that the surface defects of the graphite layer preferentially adsorbed Sodium-ions, and the attributed to the fact that the surface defects of the graphite layer preferentially adsorbed barrier of Sodium-ion repulsive force that needed to be overcome during the intercalation Sodium-ions, and the barrier of Sodium-ion repulsive force that needed to be overcome process. More importantly, as shown in Figure 9a,b, the diffusion coefficient expands during the intercalation process. More importantly, as shown in Figure 9a,b, the diffusion significantly around 50 mV, which was likely neglected in the previous characterization coefficient expands significantly around 50 mV, which was likely neglected in the pre- and calculation [70]. Therefore, a unique nanopores filling mechanism in the region of vious characterization and calculation [70]. Therefore, a unique nanopores filling mech- 0~0.05 V is proposed. anism in the region of 0~0.05 V is proposed. Figure 9. (a) GITT profile and diffusion coefficient as a function of states of charge (inset). (b) Figure 9. (a) GITT profile and diffusion coefficient as a function of states of charge (inset). (b) dQ/dV dQ/dV plot from 0.12 V to 0.01 V with corresponding diffusivity values within which a sudden plot from 0.12 V to 0.01 V with corresponding diffusivity values within which a sudden small scale small scale change can be observed. (c) Schematic of proposed Sodium-ion “adsorp- change can be observed. (c) Schematic of proposed Sodium-ion “adsorption-intercalation-pore filling” tion-intercalation-pore filling” storage mechanism [69]. (d) Schematic models of sodium storage in storage mechanism [69]. (d) Schematic models of sodium storage in HC nanopores of two different HC nanopores of two different pyrolysis temperatures [71]. pyrolysis temperatures [71]. Subsequently, the state of sodium in HC was analyzed by the combination of NMR Subsequently, the state of sodium in HC was analyzed by the combination of NMR and nitrogen adsorption [71], and the storage model of quasi-metallic sodium clusters in and nitrogen adsorption [71], and the storage model of quasi-metallic sodium clusters in nanopores was proposed in this paper (shown in Figure 9d). The pore size is limited and ◦ nthaenostpooraregse wofatshperNopa3ocsleudstienrsthoins pthaepperor(eshsouwrfanceinisFipgruevren9tded). Tathienpsuofrfeicsieiznet ipsylriomlyitseisd and theme spteorraatugreesof(ltehses tNhanc1l3u0s0terCs).oHnotwhevpeor,reatshuirgfhaecrepisyrporleyvsiesntteemdpaetraintusruefsfi,cthienctopstyoroflysis 3 tseumchpsetroartaugresin(lneasnsotphoarnes13is0t0heCd)e.cHreoawseeivnesru,raftahceigdheefercptycoronlcyesnitsratetimonpaenradtuarpelsu,ntgheicnost of sulocphesctaopragcietyi,nsnoasnuocphofriellsinigstbheehdaveicorreamsaeyinbesumrfiancimedale.fTechtecsocnhceemnattriactidoinagarnadmaopfltuhnegein adsorption-intercalation-pore filling mechanism is shown in Figure 9c; the model has slope capacity, so such filling behavior may be minimal. The schematic diagram of the since been proven and is thought to better explain most of the results [72,73]. adsorption-intercalation-pore filling mechanism is shown in Figure 9c; the model has since To sum up, the specific mechanism of sodium storage in the low-potential platform been proven and is thought to better explain most of the results [72,73]. region of HC materials is still a debatable topic. The behavior of Sodium-ion insertion, To sum up, the specific mechanism of sodium storage in the low-potential platform region of HC materials is still a debatable topic. The behavior of Sodium-ion insertion, pore adsorption and filling, as well as the deposition on the surfaces depends significantly on the complex HC microstructure. It requires more precise characterization and more specific structural regulation methods to reveal whether these behaviors occur simultaneously or independently. From the other aspect, the current mechanism of sodium storage inPDF Image | Hard Carbons as Anodes in Sodium-Ion Batteries
PDF Search Title:
Hard Carbons as Anodes in Sodium-Ion BatteriesOriginal File Name Searched:
molecules-27-06516-v2.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |