
PDF Publication Title:
Text from PDF Page: 022
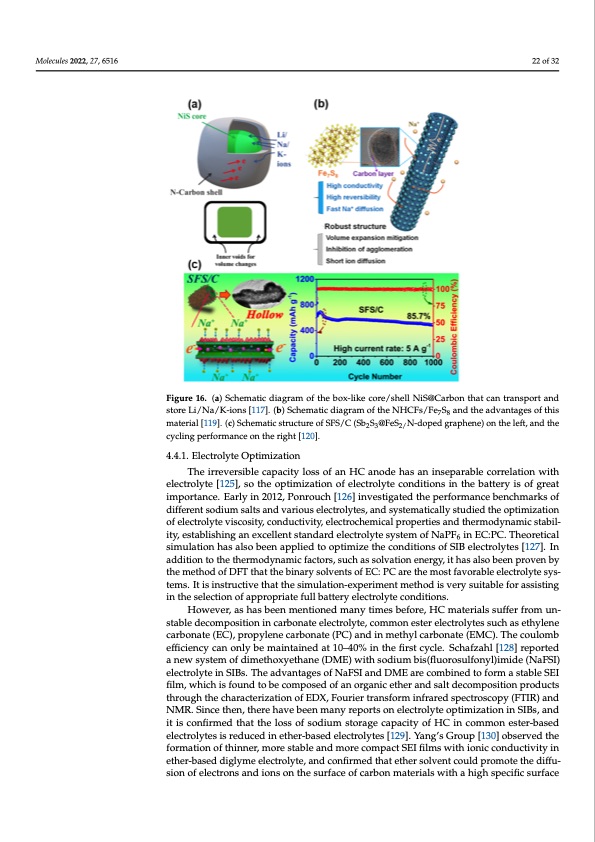
700 cycles, the capacity retention rate was nearly 100% and the rate capability of 341 mAh Molecules 2022, 27, 6516 g−1 in 5 A g−1 was obtained. It should be noted that these carbon composite materials, made by coating carbon materials onto transition metal sulfide (e.g., forming a mi- croboxed structure [122], layered nanobox structure [123] and a core-shell structure [124]), have a very different main mechanism of sodium storage and electrode reaction active substances from other HC materials mentioned in this review. What is the same is that 22 of 32 the researchers have found and utilized the advantages of carbon as an anode material for improving the electrical conductivity and mechanical properties of the materials. Figure 16. (a) Schematic diagram of the box-like core/shell NiS@Carbon that can transport and store Figure 16. (a) Schematic diagram of the box-like core/shell NiS@Carbon that can transport and Li/Na/K-ions [117]. (b) Schematic diagram of the NHCFs/Fe7S8 and the advantages of this material store Li/Na/K-ions [117]. (b) Schematic diagram of the NHCFs/Fe7S8 and the advantages of this [119]. (c) Schematic structure of SFS/C (Sb2S3@FeS2/N-doped graphene) on the left, and the cycling material [119]. (c) Schematic structure of SFS/C (Sb2S3@FeS2/N-doped graphene) on the left, and the cycling performance on the right [120]. performance on the right [120]. A carbon matrix and carbon coating were utilized to prepare a conversion-type 4.4.1. Electrolyte Optimization composite anode and showed considerable storage capacity properties, rate capability The irreversible capacity loss of an HC anode has an inseparable correlation with and cycling stability. The preparation process, industrialization, cost, and resource can- electrolyte [125], so the optimization of electrolyte conditions in the battery is of great importance. Early in 2012, Ponrouch [126] investigated the performance benchmarks of different sodium salts and various electrolytes, and systematically studied the optimization of electrolyte viscosity, conductivity, electrochemical properties and thermodynamic stabil- ity, establishing an excellent standard electrolyte system of NaPF6 in EC:PC. Theoretical simulation has also been applied to optimize the conditions of SIB electrolytes [127]. In addition to the thermodynamic factors, such as solvation energy, it has also been proven by the method of DFT that the binary solvents of EC: PC are the most favorable electrolyte sys- tems. It is instructive that the simulation-experiment method is very suitable for assisting in the selection of appropriate full battery electrolyte conditions. However, as has been mentioned many times before, HC materials suffer from un- stable decomposition in carbonate electrolyte, common ester electrolytes such as ethylene carbonate (EC), propylene carbonate (PC) and in methyl carbonate (EMC). The coulomb efficiency can only be maintained at 10–40% in the first cycle. Schafzahl [128] reported a new system of dimethoxyethane (DME) with sodium bis(fluorosulfonyl)imide (NaFSI) electrolyte in SIBs. The advantages of NaFSI and DME are combined to form a stable SEI film, which is found to be composed of an organic ether and salt decomposition products through the characterization of EDX, Fourier transform infrared spectroscopy (FTIR) and NMR. Since then, there have been many reports on electrolyte optimization in SIBs, and it is confirmed that the loss of sodium storage capacity of HC in common ester-based electrolytes is reduced in ether-based electrolytes [129]. Yang’s Group [130] observed the formation of thinner, more stable and more compact SEI films with ionic conductivity in ether-based diglyme electrolyte, and confirmed that ether solvent could promote the diffu- sion of electrons and ions on the surface of carbon materials with a high specific surfacePDF Image | Hard Carbons as Anodes in Sodium-Ion Batteries
PDF Search Title:
Hard Carbons as Anodes in Sodium-Ion BatteriesOriginal File Name Searched:
molecules-27-06516-v2.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |