
PDF Publication Title:
Text from PDF Page: 025
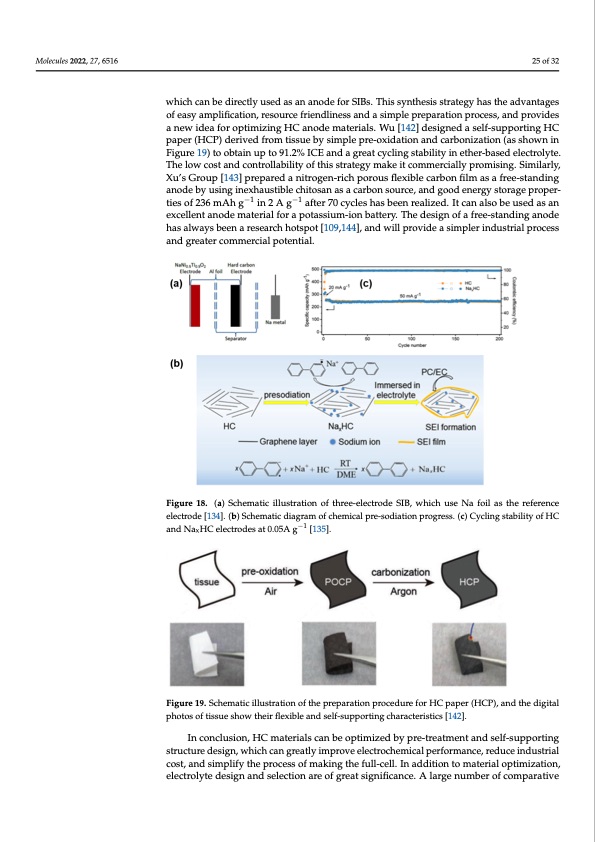
mild and efficient sodium supplement, which makes up for the problem of irreversible Molecules 2022, 27, 6516 capacity loss. Combined with an Na3V2(PO4)3 cathode to construct a full battery, it shows a high ICE of 95.0% and an energy density up to 218 Wh kg−1. As shown in Figure 18b, the pre-sodiation depth can be precisely controlled by adjusting the pre-treatment time by immerging the HC electrode in an Na-bp solution due to the strong sodiation ability of Na-bp. Xu [136] pioneered a “foreign SEI” pre-treatment strategy in which a complete25 of 32 SEI film was formed by circulating in an ester electrolyte in advance, and then the HC electrode was stabilized in the ether electrolyte. By combining the stability of an SEI film Molecules 2022, 27, x FOR PEER REVIEW −1 ties of 236 mAh g in 2 A g −1 27 of 34 after 70 cycles has been realized. It can also be used as an formed by the decomposition of the ester electrolyte with the fast transport performance which can be directly used as an anode for SIBs. This synthesis strategy has the advantages of the Sodium-ion in an ether-based electrolyte, an HC anode material with high cycling of easy amplification, resource friendliness and a simple preparation process, and provides stability and rate performance was obtained through this synergistic action. It retained a new idea for optimizing HC anode materials. Wu [142] designed a self-supporting HC 200 mAh g−1 in 0.5 A g−1 after 1000 cycles. Furthermore, to compensate for the sodium ir- paper (HCP) derived from tissue by simple pre-oxidation and carbonization (as shown in reversible capacity, a sodium-rich cathode can be used to optimize battery performance Figure 19) to obtain up to 91.2% ICE and a great cycling stability in ether-based electrolyte. [137]. Such a cathode containing sodium can provide Sodium-ions to alleviate the irre- The low cost and controllability of this strategy make it commercially promising. Similarly, versible sodium loss during cycling, thus improving the energy density of the full bat- Xu’s Group [143] prepared a nitrogen-rich porous flexible carbon film as a free-standing tery. It is also possible to use a sodium-supplement reagent (cathode sacrificial additive) anode by using inexhaustible chitosan as a carbon source, and good energy storage proper- that is oxidized as a cathode in the cycling to provide the sodium-ion capacity [138]. Ob- viously, both methods rely on metallic sodium or its active compounds (Na3N or Na3P), wexhcicehlleinetvaintaobdlyelmeaadtsertoiaslaffoertyaprotbalsesmius.mT-hioernefboartet,etrhye.iTrhcoemdmeseirgcniaolfpaotferneteia-sltiasnbdoiunngdanode thoabsealiwmaityesdbbeyensaaferteyseraisrkcsh, hanodtspaomto[1re09s,t1a4b4le],saonddiuwmilsluppropvleimdenatssitmraptelegryindeeudsstrtioalbperocess Figure 18. (a) Schematic illustration of three-electrode SIB, which use Na foil as the reference elec- eaxnpdlogrerdea.tercommercialpotential. trode [134]. (b) Schematic diagram of chemical pre-sodiation progress. (c) Cycling stability of HC and NaxHC electrodes at 0.05A g−1 [135]. 4.4.3. Self-Supporting Anode In the preparation of a full battery, the addition of a binder has a certain influence on the performance of the conductive carbon anode; for example, volume expansion in the process of charge and discharge will lead to a bad contact between the electrode and collector. In order to alleviate the inhibition of ionic diffusion and electron transfer by binders, self-supporting anodes have been studied continuously in LIBs [139]. Similarly, in recent years, self-supported anodes in SIBs without binders have been developed [140]. Yu [141] reported a simple preparation strategy for self-supporting HC anodes by constructing three-dimensional interconnected carbon nanofiber films by carbonizing bacterial cellulose, which can be directly used as an anode for SIBs. This synthesis strat- egy has the advantages of easy amplification, resource friendliness and a simple prepa- ration process, and provides a new idea for optimizing HC anode materials. Wu [142] designed a self-supporting HC paper (HCP) derived from tissue by simple pre-oxidation and carbonization (as shown in Figure 19) to obtain up to 91.2% ICE and a great cycling stability in ether-based electrolyte. The low cost and controllability of this strategy make it commercially promising. Similarly, Xu’s Group [143] prepared a nitrogen-rich porous flexible carbon film as a free-standing anode by using inexhaustible chitosan as a carbon source, and good energy storage properties of 236 mAh g−1 in 2 A g−1 after 70 cycles has bFeiegnurea1l8iz. ed(a.)ItSchanemaalstoicbiellusterdatiaosnanofetxhcrelele-enltecatnrode mSIaBt,ewriahlicfhoruasepoNtassfoiuilma-siotnhebaret-ference telreyc.trTohde [d1e3s4i]g.n(bo)fSachfermeea-tsitcadnidaginragmanoofdcheehmaiscallpwrae-yssodbieaetnionaprerosgearerscsh. (hco)tCsypcoltin[g10st9a,1b4il4it]y, of HC −1 and will provide a simpler industrial process and greater commercial potential. and NaxHC electrodes at 0.05A g [135]. Figure 19. Schematic illustration of the preparation procedure for HC paper (HCP), and the digital Figure 19. Schematic illustration of the preparation procedure for HC paper (HCP), and the digital pphhoototossoofftitsisuseueshsohwowthtehirefilreflxiebxliebalendansedlfs-esulfp-spuoprptinogrticnhgarcahcaterraicstiecrsis[t1i4c2s][.142]. In conclusion, HC materials can be optimized by pre-treatment and self-supporting In conclusion, HC materials can be optimized by pre-treatment and self-supporting ssttrruucctutureredessiignn,,whiich can greatly iimprroveeeleleccttrroocchemiicalppeerrffoorrmance,rreedduucceeinind-ustrial dcuostr,ialncdosti,mapnldifsyimthpelipfyrotchespsrofcmesaskoifnmgathkienfgutlhl-ecefulll.l-Icnelald. Idnitaidonditoiomn taotemriatleoripatliompi-zation, teimleicztartoiolynt,eedleecstirgonlyatendeseiglenctainodnsaerlecotfiognreaartesoigfngirfieactansicgen.iAficlaanrcgee.Anulmarbgernoufmcobemrpoafrative comparative experiments are designed to select the appropriate electrolyte system to improve battery performance. Furthermore, theoretical studies have confirmed the in- fluence of electrolyte on electrode material properties [145]. For the ether-based electro- lyte mentioned above, the fast sodium storage kinetics of HC in ether-based electrolyte can be attributed to three aspects: (i) The migration rate of Sodium-ions in ether-basedPDF Image | Hard Carbons as Anodes in Sodium-Ion Batteries
PDF Search Title:
Hard Carbons as Anodes in Sodium-Ion BatteriesOriginal File Name Searched:
molecules-27-06516-v2.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |