
PDF Publication Title:
Text from PDF Page: 004
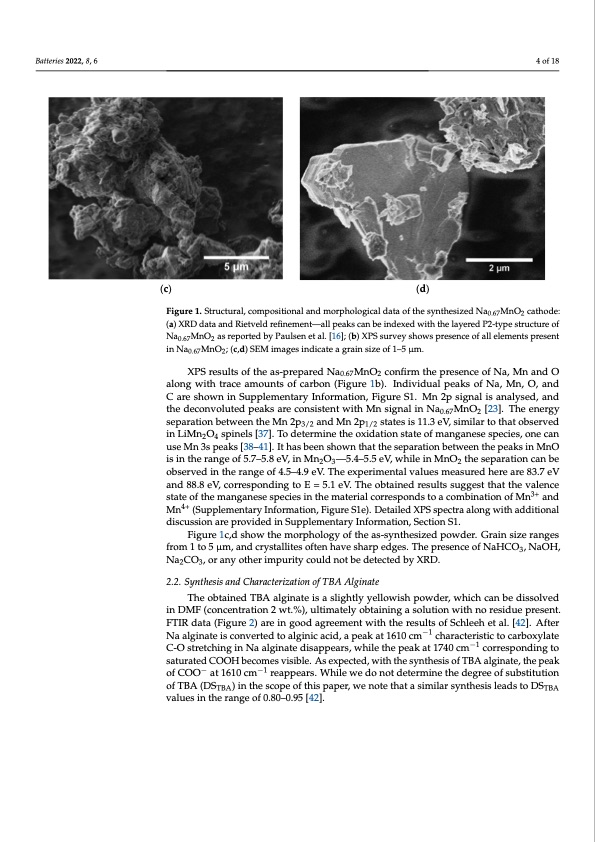
BatteBraietste2ri0e2s2,082,2x,8F,O6RPEERREVIEW 44ofo1f819 (c) (d) Figure 1. Structural, compositional and morphological data of the synthesized Na0.67MnO2 cathode: Figure 1. Structural, compositional and morphological data of the synthesized Na0.67MnO2 cathode: (a) XRD data and Rietveld refinement—all peaks can be indexed with the layered P2-type structure (a) XRD data and Rietveld refinement—all peaks can be indexed with the layered P2-type structure of of Na0.67MnO2 as reported by Paulsen et al. [16]; (b) XPS survey shows presence of all elements pre- Na0.67MnO2 as reported by Paulsen et al. [16]; (b) XPS survey shows presence of all elements present sent in Na0.67MnO2; (c,d) SEM images indicate a grain size of 1–5 μm. in Na0.67MnO2; (c,d) SEM images indicate a grain size of 1–5 μm. XPS results of the as-prepared Na0.67MnO2 confirm the presence of Na, Mn and O XPS results of the as-prepared Na0.67MnO2 confirm the presence of Na, Mn and O aloanlognwgiwthitthratcreacaemaomuonutsntosfocfarcbaorbno(nFi(gFuigreur1eb)1.bI)n.dIinvdidivuiadlupaelapkesaokfsNofa,NMa,nM,On,,aOn,daCndare shCowanreinshSouwpnplienmSeunptpalreymInenfotarmryaItniofonr,mFiagtuiorne,SF1i.gMurne2Sp1.siMgnal2ips asinganlyalseisd,aannaldysthede,daencdon- votlhuetedecpoenavkosluatredcpoenaskisteanretcwointhsisMtentswiginthalMinNsigan0.6a7lMinON2a[02.637M].nTOhe2[e2n3e]r.gTyhseeepnaerragtyion separation between the Mn 2p and Mn 2p states is 11.3 eV, similar to that observed between the Mn 2p3/2 and Mn 23p/21/2 states is 111/.23 eV, similar to that observed in LiMn2O4 inLiMnO spinels[37].Todeterminetheoxidationstateofmanganesespecies,onecan spinels [372]. 4To determine the oxidation state of manganese species, one can use Mn 3s use Mn 3s peaks [38–41]. It has been shown that the separation between the peaks in MnO peaks [38–41]. It has been shown that the separation between the peaks in MnO is in the is in the range of 5.7–5.8 eV, in Mn2O3—5.4–5.5 eV, while in MnO2 the separation can be range of 5.7–5.8 eV, in Mn2O3—5.4–5.5 eV, while in MnO2 the separation can be observed observed in the range of 4.5–4.9 eV. The experimental values measured here are 83.7 eV in the range of 4.5–4.9 eV. The experimental values measured here are 83.7 eV and 88.8 and 88.8 eV, corresponding to E = 5.1 eV. The obtained results suggest that the valence eV, corresponding to E = 5.1 eV. The obtained results suggest that the valence state of the state of the manganese species in the material corresponds to a combination of Mn3+ and manganese species in the material corresponds to a combination of Mn3+ and Mn4+ (Sup- Mn4+ (Supplementary Information, Figure S1e). Detailed XPS spectra along with additional plementary Information, Figure S1e). Detailed XPS spectra along with additional discus- discussion are provided in Supplementary Information, Section S1. sion are provided in Supplementary Information, Section S1. Figure 1c,d show the morphology of the as-synthesized powder. Grain size ranges Figure 1c,d show the morphology of the as-synthesized powder. Grain size ranges from 1 to 5 μm, and crystallites often have sharp edges. The presence of NaHCO3, NaOH, from 1 to 5 μm, and crystallites often have sharp edges. The presence of NaHCO3, NaOH, Na2CO3, or any other impurity could not be detected by XRD. Na2CO3, or any other impurity could not be detected by XRD. 2.2. Synthesis and Characterization of TBA Alginate 2.2. SynTthesoisbatanidneCdhaTrBaActearligzaintiaotne oifs TaBsAligAhltglyinyaetellowish powder, which can be dissolved in DMF (concentration 2 wt.%), ultimately obtaining a solution with no residue present. The obtained TBA alginate is a slightly yellowish powder, which can be dissolved in FTIR data (Figure 2) are in good agreement with the results of Schleeh et al. [42]. After DMF (concentration 2 wt.%), ultimately obtaining a solution with no residue present. FTIR Na alginate is converted to alginic acid, a peak at 1610 cm−1 characteristic to carboxylate data (Figure 2) are in good agreement with the results of Schleeh et al. [42]. After Na algi- C-O stretching in Na alginate disappears, while the peak at 1740 cm−1 corresponding to nate is converted to alginic acid, a peak at 1610 cm−1 characteristic to carboxylate C-O saturated COOH becomes visible. As expected, with the synthesis of TBA alginate, the peak stretching in Na alginate disappears, while the peak at 1740 cm−1 corresponding to satu- of COO− at 1610 cm−1 reappears. While we do not determine the degree of substitution rated COOH becomes visible. As expected, with the synthesis of TBA alginate, the peak of TBA (DSTBA) in the scope of this paper, we note that a similar synthesis leads to DSTBA of COO− at 1610 cm−1 reappears. While we do not determine the degree of substitution of values in the range of 0.80–0.95 [42]. TBA (DSTBA) in the scope of this paper, we note that a similar synthesis leads to DSTBA values in the range of 0.80–0.95 [42].PDF Image | Na-Ion Batteries Tetrabutylammonium Alginate Binder
PDF Search Title:
Na-Ion Batteries Tetrabutylammonium Alginate BinderOriginal File Name Searched:
batteries-08-00006.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |