
PDF Publication Title:
Text from PDF Page: 003
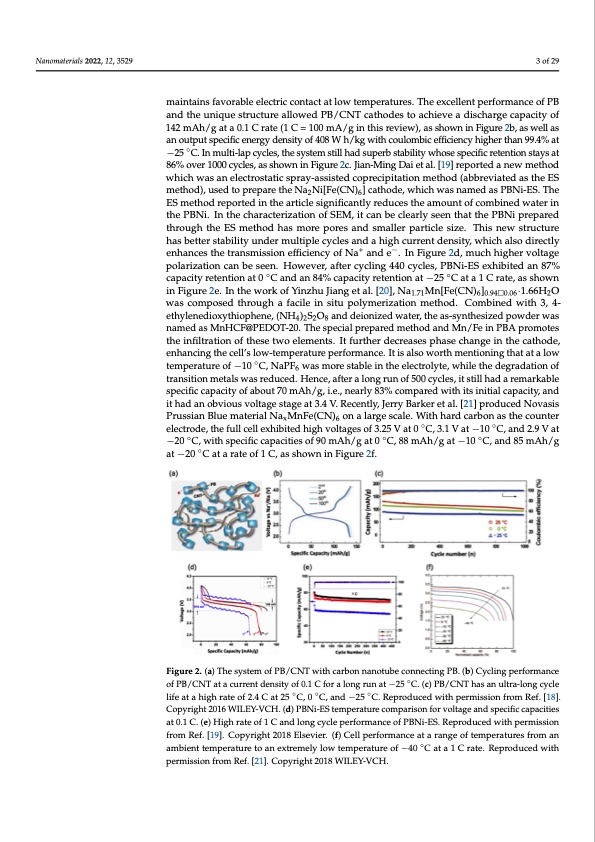
Nanomaterials 2022, 12, 3529 3 of 28 Nanomaterials 2022, 12, 3529 2a. The material was demonstrated to have fast and stable cycling performance at −25 °C. At the same time, PB crystals maintain a small volume change during the Na+ insertion and extraction process. In addition, a larger lattice parameter of the perovskite framework of PB reduces the activation energy of sodium-ion diffusion, and the concatenate of CNT in PB nanocrystals maintains favorable electric contact at low temperatures. The excellent performance of PB and the unique structure allowed PB/CNT cathodes to achieve a dis- and the unique structure allowed PB/CNT cathodes to achieve a discharge capacity of charge capacity of 142 mAh/g at a 0.1 C rate (1 C = 100 mA/g in this review), as shown in 142 mAh/g at a 0.1 C rate (1 C = 100 mA/g in this review), as shown in Figure 2b, as well as Figure 2b, as well as an output specific energy density of 408 W h/kg with coulombic effi- ciency higher than 99.4% at −25 °C. In multi-lap cycles, the system still had superb stability 3 of 29 maintains favorable electric contact at low temperatures. The excellent performance of PB an output specific energy density of 408 W h/kg with coulombic efficiency higher than 99.4% at ◦ −25 C.Inmulti-lapcycles,thesystemstillhadsuperbstabilitywhosespecificretentionstaysat whose specific retention stays at 86% over 1000 cycles, as shown in Figure 2c. Jian-Ming 86% over 1000 cycles, as shown in Figure 2c. Jian-Ming Dai et al. [19] reported a new method Dai et al. [19] reported a new method which was an electrostatic spray-assisted coprecip- witahtiochnmweatshaonde(alebcbtreovsitatteidcaspsrthaye-EaSssmisettehdodco),purseecdiptiotaptrioepnamreettheoNda(2aNbib[Frev(CiaNte)6d]caasthth-eES omdet,hwodh)ic,huswedastonapmreepdaraestPhBeNi-aE2SN.iT[Fhe(CENS)m6]etchaothdordeep,owrtheidchinwtahsenaartmiceledsaisgnPiBfiNcain-EtlSy.The rEeSdumcetshtohde arempourntetdofincotmhebianretdiclweastiegrniinfitchaenPtlByNrei.dIunctehsetchearaamctoeurinzattoiofncomf SbEiMne,ditwcanter in btheeclPeaBrNlyi.seIennttheatcthaerPaBctNeriipzraetpioanreodfthSrEoMug, hitthcaenESbemceltehaordlyhasseemnotrheaptotrhees aPnBdNsmi parlleeprared ptharotiuclgehsitzhee. TEhSismnewthsotdruhctausremhoarsebpetotererstanbdilitsymuanldler pmaurlttiicpllee sciyzcel.esTahnids anheiwghsctruur-cture +− rheanstbdetntesrityst,awbhilictyh ualnsdoedrirmecutltyipelnehcaynclestahnedtranhsigmhiscsuiornreenftfidciensciytyo,fwNhaichanadlsoe .dIinrectly +− Fenighuarnec2eds,tmheucthrahnisgmheisrsvionltaegfeficpioelnacriyzaotfioNnacananbde seee.nI.nHFoiwgeuvrer2, daf,temruccyhclihnigh44e0r vcyo-ltage cles, PBNi-ES exhibited an 87% capacity retention at 0 °C and an 84% capacity retention polarization can be seen. However, after cycling 440 cycles, PBNi-ES exhibited an 87% at −25 °C at a 1 C rate, as shown in Figure 2e. In the work of Yinzhu Jiang et al. [20], capacity retention at 0 ◦C and an 84% capacity retention at −25 ◦C at a 1 C rate, as shown Na1.71Mn[Fe(CN)6]0.94□0.06·1.66H2O was composed through a facile in situ polymerization in Figure 2e. In the work of Yinzhu Jiang et al. [20], Na1.71Mn[Fe(CN)6]0.940.06·1.66H2O method. Combined with 3, 4-ethylenedioxythiophene, (NH4)2S2O8 and deionized water, was composed through a facile in situ polymerization method. Combined with 3, 4- the as-synthesized powder was named as MnHCF@PEDOT-20. The special prepared ethylenedioxythiophene, (NH4)2S2O8 and deionized water, the as-synthesized powder was method and Mn/Fe in PBA promotes the infiltration of these two elements. It further de- named as MnHCF@PEDOT-20. The special prepared method and Mn/Fe in PBA promotes creases phase change in the cathode, enhancing the cell’s low-temperature performance. the infiltration of these two elements. It further decreases phase change in the cathode, It is also worth mentioning that at a low temperature of −10 °C, NaPF6 was more stable in enhancing the cell’s low-temperature performance. It is also worth mentioning that at a low the electrolyte, while ◦the degradation of transition metals was reduced. Hence, after a long temperature of −10 C, NaPF6 was more stable in the electrolyte, while the degradation of run of 500 cycles, it still had a remarkable specific capacity of about 70 mAh/g, i.e., nearly transition metals was reduced. Hence, after a long run of 500 cycles, it still had a remarkable 83% compared with its initial capacity, and it had an obvious voltage stage at 3.4 V. Re- specific capacity of about 70 mAh/g, i.e., nearly 83% compared with its initial capacity, and cently, Jerry Barker et al. [21] produced Novasis Prussian Blue material NaxMnFe(CN)6 on it had an obvious voltage stage at 3.4 V. Recently, Jerry Barker et al. [21] produced Novasis a large scale. With hard carbon as the counter electrode, the full cell exhibited high volt- Prussian Blue material NaxMnFe(CN)6 on a large scale. With hard carbon as the counter ages of 3.25 V at 0 °C, 3.1 V at −10 °C, and 2.9 V at −20 °C, with specific capacities of 90 electrode, the full cell exhibited high voltages of 3.25 V at 0 ◦C, 3.1 V at −10 ◦C, and 2.9 V at mAh/g at 0 °C, 88 mAh/g at −10 °C, and 85 mAh/g at −20 °C at a rate of 1 C, as shown in −20 ◦C, with specific capacities of 90 mAh/g at 0 ◦C, 88 mAh/g at −10 ◦C, and 85 mAh/g Figure 2f. at −20 ◦C at a rate of 1 C, as shown in Figure 2f. Figure2..((aa))TThheessyystsetmemofofPPB/BC/NCTNwTiwthitcharcbaornbonnanoatnuobteucboencnoenctninecgtiPnBg.(PbB).C(byc)lCinygclpinergfopremrfaonrcmeance ◦ ofPB/CCNNTTaattaacucurrernenttddenesnistyityofo0f.01.1CCfofroarlaonlognrgurnuanta−t25−°2C5. (Cc).P(cB)/CPBN/TChNasTahnauslatrnau-llotnrag-lcoyncglecycle life at a high rate of 2.4 C at 25 °C◦ , 0 °C◦, and −25 °C.◦Reproduced with permission from Ref. [18]. life at a high rate of 2.4 C at 25 C, 0 C, and −25 C. Reproduced with permission from Ref. [18]. Copyright 2016 WILEY-VCH. (d) PBNi-ES temperature comparison for voltage and specific capacities at 0.1 C. (e) High rate of 1 C and long cycle performance of PBNi-ES. Reproduced with permission from Ref. [19]. Copyright 2018 Elsevier. (f) Cell performance at a range of temperatures from an ambient temperature to an extremely low temperature of −40 ◦C at a 1 C rate. Reproduced with permission from Ref. [21]. Copyright 2018 WILEY-VCH.PDF Image | Na Ion Batteries Used at Low Temperatures
PDF Search Title:
Na Ion Batteries Used at Low TemperaturesOriginal File Name Searched:
nanomaterials-12-03529-v4.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |