
PDF Publication Title:
Text from PDF Page: 010
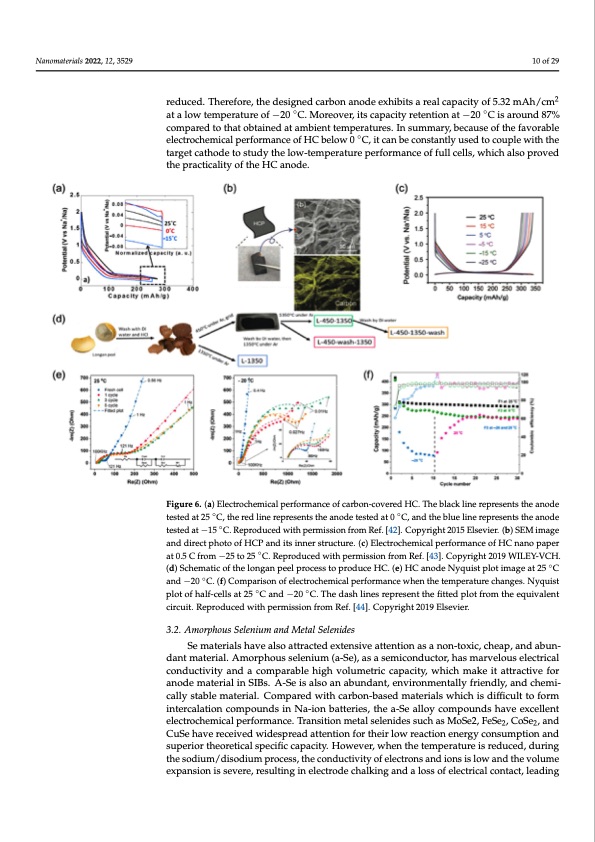
Nanomaterials 2022, 12, 3529 Nanomaterials 2022, 12, 3529 10 of 28 10 of 29 been proposed, which effectively reduced pore diameter from more than 1 nm to less than 0.5 nm. In this way, interfacial contact between the electrolyte and pores could be reduced. 22 Therefore, the designed carbon anode exhibits a real capacity of 5.32 mAh/cm at a low reduced. Therefore, the designed carbon anode exhibits a real capacity of 5.32 mAh/cm temperature of −20 °C. Moreover, its capacity retention at −20 °C is around 87% compared at a low temperature of −20 ◦C. Moreover, its capacity retention at −20 ◦C is around 87% to that obtained at ambient temperatures. In summary, because of the favorable electro- compared to that obtained at ambient temperatures. In summary, because of the favorable cehlecmtroiccahlepmericfoarlmpearnfocermofaHncCe obfelHowC b0e°lCow, it0caCn,biet caonsbteancotlnysutasnetdlytoucsoeduptolecwouitphlethweitahrgtheet ctartgheotdceathoosdtuedtoystthuedlyotwh-etelomwp-etreamtuprerapteurfeorpmerafnocrmeoanfcfeulolfcfeullsl,cewllhsi,cwhhailcshoaplsrovperdovtehde pthreacptircaacltitcyaloitfythoefHthCeHanCodaneo.de. ◦ Figure 6. (a) Electrochemical performance of carbon-covered HC. The black line represents the an- Figure 6. (a) Electrochemical performance of carbon-covered HC. The black line represents the anode ode tested at 25 °C, the red line represents the anode tested at 0 °C, and the blue line represents the tested at 25 ◦C, the red line represents the anode tested at 0 ◦C, and the blue line represents the anode anode tested at −15 °C. Reproduced with permission from Ref. [42]. Copyright 2015 Elsevier. (b) tested at −15 ◦C. Reproduced with permission from Ref. [42]. Copyright 2015 Elsevier. (b) SEM image SEM image and direct photo of HCP and its inner structure. (c) Electrochemical performance of HC and direct photo of HCP and its inner structure. (c) Electrochemical performance of HC nano paper nano paper at 0.5 C from −25 to 25 °C. Reproduced with permission from Ref. [43]. Copyright 2019 ◦ at 0.5 C from −25 to 25 C. Reproduced with permission from Ref. [43]. Copyright 2019 WILEY-VCH. WILEY-VCH. (d) Schematic of the longan peel process to produce HC. (e) HC anode Nyquist plot i(md)aSgcehaetm2a5ti°cCofanthde−lo20ng°aCn. (pfe)eClopmropcaersissotonporfoedluecteroHcChe. m(e)icHalCpaenrfodrme Nanycqeuwisht epnlothiemtaegmepaetr2a5turCe ◦ cahnadn−ge2s0.NCy.q(fu)isCtopmloptaorfishoanlfo-fcellescatrto2c5he°mCiacnadlp−e2r0fo°rCm.aTnhcedwahshenlitnhestreemppresreantutrtehechfaitntegdesp.lNotyfqruoimst the equivalent circuit. R◦ eproduced ◦with permission from Ref. [44]. Copyright 2019 Elsevier. plot of half-cells at 25 C and −20 C. The dash lines represent the fitted plot from the equivalent circuit. Reproduced with permission from Ref. [44]. Copyright 2019 Elsevier. 3.2. Amorphous Selenium and Metal Selenides Se materials have also attracted extensive attention as a non-toxic, cheap, and abun- dant Smeamteartiearl.iaAlsmhoarvpehaolusso saetlteranciutemd e(ax-tSeen)s,iavseatstemntiicondasuactnoro,nh-taosxmica, rcvhelaopu,saenldecatrbiucanl- cdoandt umctaitveirtiyala.nAdmaocropmhpouarsasbellenhiughmv(oal-uSme),etarsicacsaepmaiccitoyn,dwuhcitcohr, mhaskemiatravtetrlaocutsiveelefcotriacna-l ocodnedmuacteivriatyl iannSdIBas.cAom-Speairsaabllseo hainghabvuonlduamnet,treincvciaropnamciteyn, twallhyicfhriemndaklye, iatnadttcrhaectmiviceaflolyr satnaobdlemaatteerriaial.l CinomSIpBas.reAd-wSeitihs caalsrbooan-baabsuenddmanate, reinavlsirwonhimchenistadlliyffifcruieltntdolyfo, ramndinctheermcai-- lcatliloynsctaobmlepomuantdersiainl. NCao-miopnabreadttewrieths, ctahrebao-nS-ebaslleody mcoamteprioaulsndwshhicahveisedxicfefilcleunlt teolefcotrom- cinhteemrciaclaaltpioenrfocormapnocuen.dTsrainsiNtiao-niomnebtalttserliensi,dtehsesau-cSheasllMoyoSceo2m,FpeoSuen2,dCsohSaev2,eaenxdceCllueSnet helaevcetrroeccheeimveidcawl pideerfsoprrmeadncaet.teTnrtainosnitfiorntmheitraloswelerneaidcteisosnuecnhearsgyMcoSnes2u,mFepSteio2,nCaonSde2s,uapned- rCiourSethheaovretriceacleisvpedciwficidceasparceiatyd. aHttoewnteiovnerf,owr htheenirthloewtermeapcetriaotnuereneisrgryedcuocnesdu,mdputriionngatnhde soudpieurmio/rdtihseoodrieutmicaplrsopceecsisfi,cthcaepcaocnitdyu.cHtiovwiteyvoefr,ewlehcternonthseatnedmipoenrsatiusrleowisraendutcheed,vdoulurimnge the sodium/disodium process, the conductivity of electrons and ions is low and the volume expansion is severe, resulting in electrode chalking and a loss of electrical contact, leading 3.2. Amorphous Selenium and Metal Selenides ◦PDF Image | Na Ion Batteries Used at Low Temperatures
PDF Search Title:
Na Ion Batteries Used at Low TemperaturesOriginal File Name Searched:
nanomaterials-12-03529-v4.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |