
PDF Publication Title:
Text from PDF Page: 021
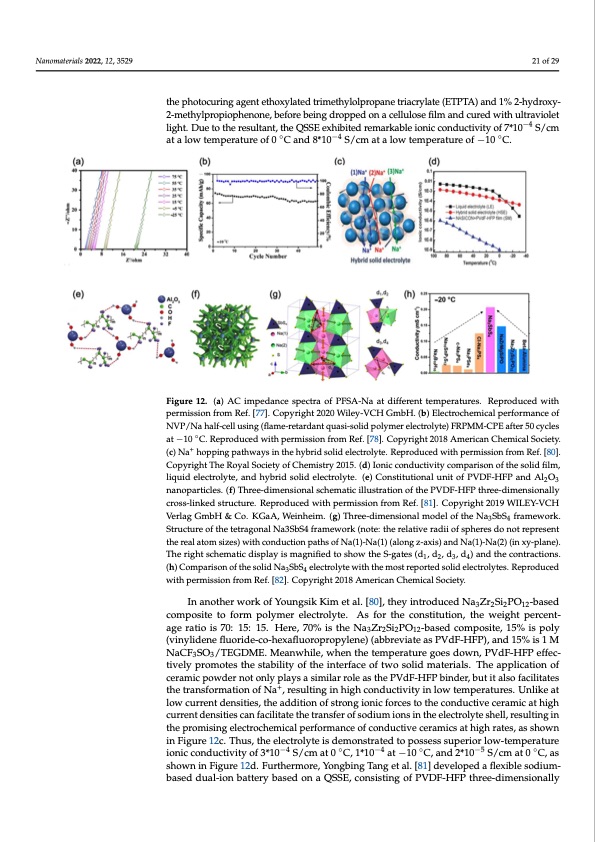
QSSE facilitates the fast ionic migration of both anions and cations. Thus, sandwiched by the graphite cathode and the Sn anode, the full cell showed specific capacities of 65 mAh/g at −5 °C and 45 mAh/g at −20 °C with rate of 5 C. Moreover, Chengdu Liang et al. [82] devised a 3D superionic conductor Na3SbS4 solid electrolyte model to investigate a con- Nanomaterials 2022, 12, 3529 ductive mechanism, as shown in Figure 12g. Because of the great structural stability with the 3D conductive tunnel network, the ionic conductivity would only slightly drop in an extreme low-temperature environment. Therefore, at −20 °C, Na3SbS4 was reported to 21 of 29 the photocuring agent ethoxylated trimethylolpropane triacrylate (ETPTA) and 1% 2-hydroxy- −4 hold an ionic condu2c-tmiveitthyloprfo2p*io1p0henSo/ncem, b,ewforheibcehinigsdtrhoeppheidgohneastceallmulosnegfiltmheanmd ocusrtedrewpiothrtueldtraviolet −4 light. Due to the resultant, the QSSE exhibited remarkable ionic conductivity of 7*10 S/cm solid electrolytes, as shown in Figure 12h. at a low temperature of 0 ◦C and 8*10−4 S/cm at a low temperature of −10 ◦C. Figure 12. (a) AC impedance spectra of PFSA-Na at different temperatures. Reproduced with Figure 12. (a) AC impepderamnicsesiosnpefrcotmraRoef.P[7F7S].AC-oNpyariagthdt 2if0f2e0rWenilteyte-VmCpHerGamtubrHe.s(.bR) Eeplercotrdocuhceemdicwaliptherfpoermr-ance of mission from Ref. [77N].VCP/oNpyarhiaglfh-ctel2l0u2si0ngW(flialmeye-rVetCarHdanGtmqubasHi-s.o(lbid)pEollyemcteroeclehcetrmolyictea)lFRpPeMrfMor-mCPaEnacfetero5f0cycles ◦ NVP/Na half-cell usingat(−fl1a0mCe-.rReetparodauncetdqwuaitship-seormlidisspionlyfrmomerRefl.e[c7t8r]o. lCyotpey)rFigRhPt 2M01M8 A-CmPeEricafnteCrh5em0icyal-Society. (c) Na+ hopping pathways in the hybrid solid electrolyte. Reproduced with permission from Ref. [80]. cles at −10 °C. Reproduced with permission from Ref. [78]. Copyright 2018 American Chemical So- + Copyright The Royal Society of Chemistry 2015. (d) Ionic conductivity comparison of the solid film, ciety. (c) Na hopping pathways in the hybrid solid electrolyte. Reproduced with permission from liquid electrolyte, and hybrid solid electrolyte. (e) Constitutional unit of PVDF-HFP and Al2O3 Ref. [80]. Copyright The Royal Society of Chemistry 2015. (d) Ionic conductivity comparison of the nanoparticles. (f) Three-dimensional schematic illustration of the PVDF-HFP three-dimensionally solid film, liquid electrolyte, and hybrid solid electrolyte. (e) Constitutional unit of PVDF-HFP and cross-linked structure. Reproduced with permission from Ref. [81]. Copyright 2019 WILEY-VCH Al2O3 nanoparticles. (f) Three-dimensional schematic illustration of the PVDF-HFP three-dimen- Verlag GmbH & Co. KGaA, Weinheim. (g) Three-dimensional model of the Na SbS framework. sionally cross-linked structure. Reproduced with permission from Ref. [81]. Copyright 2019 WILEY- Structure of the tetragonal Na3SbS4 framework (note: the relative radii of spheres do not represent VCH Verlag GmbH & Co. KGaA, Weinheim. (g) Three-dimensional model of the Na3SbS4 frame- the real atom sizes) with conduction paths of Na(1)-Na(1) (along z-axis) and Na(1)-Na(2) (in xy-plane). work. Structure of the tetragonal Na3SbS4 framework (note: the relative radii of spheres do not The right schematic display is magnified to show the S-gates (d1, d2, d3, d4) and the contractions. represent the real atom sizes) with conduction paths of Na(1)-Na(1) (along z-axis) and Na(1)-Na(2) (h) Comparison of the solid Na3SbS4 electrolyte with the most reported solid electrolytes. Reproduced (in xy-plane). The right schematic display is magnified to show the S-gates (d1, d2, d3, d4) and the with permission from Ref. [82]. Copyright 2018 American Chemical Society. contractions. (h) Comparison of the solid Na3SbS4 electrolyte with the most reported solid electro- In another work of Youngsik Kim et al. [80], they introduced Na3Zr2Si2PO12-based lytes. Reproduced with permission from Ref. [82]. Copyright 2018 American Chemical Society. composite to form polymer electrolyte. As for the constitution, the weight percent- age ratio is 70: 15: 15. Here, 70% is the Na Zr Si PO -based composite, 15% is poly 3 2 2 12 In the end of this section, we list the electrolytes mentioned above in Table 1. Table 1 (vinylidene fluoride-co-hexafluoropropylene) (abbreviate as PVdF-HFP), and 15% is 1 M compares the conductivities of all the above-mentioned electrolytes at low temperatures, NaCF3SO3/TEGDME. Meanwhile, when the temperature goes down, PVdF-HFP effec- the specific capacitietisv,ealynpdrotmheotensutmhebsetarboilfitybaotfttehreyinctyecrflaecse.oEfltewctorosolylitdemsatlewrialys.sTehaesialpypulicna-tionof ceramic powder not only plays a similar role as the PVdF-HFP binder, but it also facilitates dergo phase change and more complex interface evolution at low temperatures, leading the transformation of Na+, resulting in high conductivity in low temperatures. Unlike at to a decrease in the cycle stability of the battery. Therefore, the number of working cycles low current densities, the addition of strong ionic forces to the conductive ceramic at high of the battery was taken as an important index. As the research proceeds, a combination current densities can facilitate the transfer of sodium ions in the electrolyte shell, resulting in the promising electrochemical performance of conductive ceramics at high rates, as shown in Figure 12c. Thus, the electrolyte is demonstrated to possess superior low-temperature ionic conductivity of 3*10−4 S/cm at 0 ◦C, 1*10−4 at −10 ◦C, and 2*10−5 S/cm at 0 ◦C, as shown in Figure 12d. Furthermore, Yongbing Tang et al. [81] developed a flexible sodium- based dual-ion battery based on a QSSE, consisting of PVDF-HFP three-dimensionally 34PDF Image | Na Ion Batteries Used at Low Temperatures
PDF Search Title:
Na Ion Batteries Used at Low TemperaturesOriginal File Name Searched:
nanomaterials-12-03529-v4.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |