
PDF Publication Title:
Text from PDF Page: 005
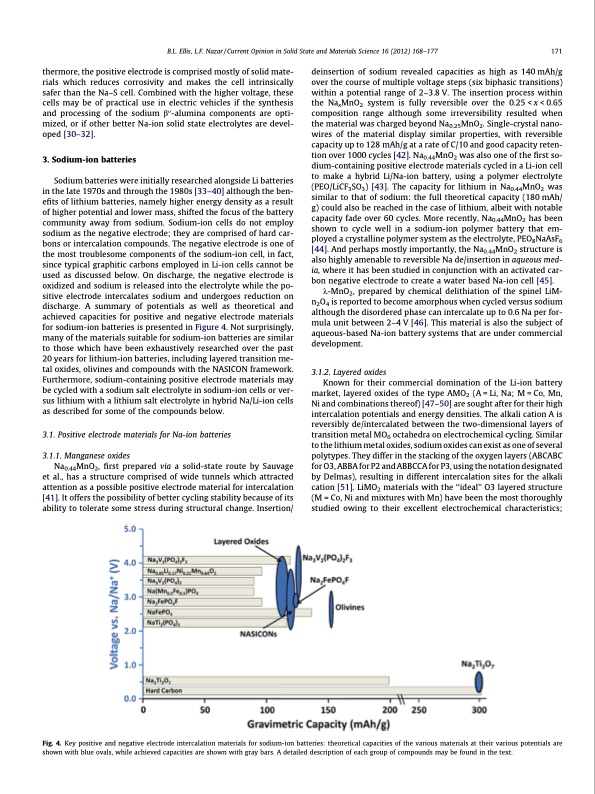
B.L. Ellis, L.F. Nazar / Current Opinion in Solid State and Materials Science 16 (2012) 168–177 171 thermore, the positive electrode is comprised mostly of solid mate- rials which reduces corrosivity and makes the cell intrinsically safer than the Na–S cell. Combined with the higher voltage, these cells may be of practical use in electric vehicles if the synthesis and processing of the sodium b00-alumina components are opti- mized, or if other better Na-ion solid state electrolytes are devel- oped [30–32]. 3. Sodium-ion batteries Sodium batteries were initially researched alongside Li batteries in the late 1970s and through the 1980s [33–40] although the ben- efits of lithium batteries, namely higher energy density as a result of higher potential and lower mass, shifted the focus of the battery community away from sodium. Sodium-ion cells do not employ sodium as the negative electrode; they are comprised of hard car- bons or intercalation compounds. The negative electrode is one of the most troublesome components of the sodium-ion cell, in fact, since typical graphitic carbons employed in Li-ion cells cannot be used as discussed below. On discharge, the negative electrode is oxidized and sodium is released into the electrolyte while the po- sitive electrode intercalates sodium and undergoes reduction on discharge. A summary of potentials as well as theoretical and achieved capacities for positive and negative electrode materials for sodium-ion batteries is presented in Figure 4. Not surprisingly, many of the materials suitable for sodium-ion batteries are similar to those which have been exhaustively researched over the past 20 years for lithium-ion batteries, including layered transition me- tal oxides, olivines and compounds with the NASICON framework. Furthermore, sodium-containing positive electrode materials may be cycled with a sodium salt electrolyte in sodium-ion cells or ver- sus lithium with a lithium salt electrolyte in hybrid Na/Li-ion cells as described for some of the compounds below. 3.1. Positive electrode materials for Na-ion batteries 3.1.1. Manganese oxides Na0.44MnO2, first prepared via a solid-state route by Sauvage et al., has a structure comprised of wide tunnels which attracted attention as a possible positive electrode material for intercalation [41]. It offers the possibility of better cycling stability because of its ability to tolerate some stress during structural change. Insertion/ deinsertion of sodium revealed capacities as high as 140 mAh/g over the course of multiple voltage steps (six biphasic transitions) within a potential range of 2–3.8 V. The insertion process within the NaxMnO2 system is fully reversible over the 0.25 < x < 0.65 composition range although some irreversibility resulted when the material was charged beyond Na0.25MnO2. Single-crystal nano- wires of the material display similar properties, with reversible capacity up to 128 mAh/g at a rate of C/10 and good capacity reten- tion over 1000 cycles [42]. Na0.44MnO2 was also one of the first so- dium-containing positive electrode materials cycled in a Li-ion cell to make a hybrid Li/Na-ion battery, using a polymer electrolyte (PEO/LiCF3SO3) [43]. The capacity for lithium in Na0.44MnO2 was similar to that of sodium: the full theoretical capacity (180 mAh/ g) could also be reached in the case of lithium, albeit with notable capacity fade over 60 cycles. More recently, Na0.44MnO2 has been shown to cycle well in a sodium-ion polymer battery that em- ployed a crystalline polymer system as the electrolyte, PEO8NaAsF6 [44]. And perhaps mostly importantly, the Na0.44MnO2 structure is also highly amenable to reversible Na de/insertion in aqueous med- ia, where it has been studied in conjunction with an activated car- bon negative electrode to create a water based Na-ion cell [45]. k-MnO2, prepared by chemical delithiation of the spinel LiM- n2O4 is reported to become amorphous when cycled versus sodium although the disordered phase can intercalate up to 0.6 Na per for- mula unit between 2–4 V [46]. This material is also the subject of aqueous-based Na-ion battery systems that are under commercial development. 3.1.2. Layered oxides Known for their commercial domination of the Li-ion battery market, layered oxides of the type AMO2 (A = Li, Na; M = Co, Mn, Ni and combinations thereof) [47–50] are sought after for their high intercalation potentials and energy densities. The alkali cation A is reversibly de/intercalated between the two-dimensional layers of transition metal MO6 octahedra on electrochemical cycling. Similar to the lithium metal oxides, sodium oxides can exist as one of several polytypes. They differ in the stacking of the oxygen layers (ABCABC for O3, ABBA for P2 and ABBCCA for P3, using the notation designated by Delmas), resulting in different intercalation sites for the alkali cation [51]. LiMO2 materials with the ‘‘ideal’’ O3 layered structure (M = Co, Ni and mixtures with Mn) have been the most thoroughly studied owing to their excellent electrochemical characteristics; Fig. 4. Key positive and negative electrode intercalation materials for sodium-ion batteries: theoretical capacities of the various materials at their various potentials are shown with blue ovals, while achieved capacities are shown with gray bars. A detailed description of each group of compounds may be found in the text.PDF Image | Sodium and sodium-ion energy storage batteries
PDF Search Title:
Sodium and sodium-ion energy storage batteriesOriginal File Name Searched:
2012_Na-battery_review.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |