
PDF Publication Title:
Text from PDF Page: 008
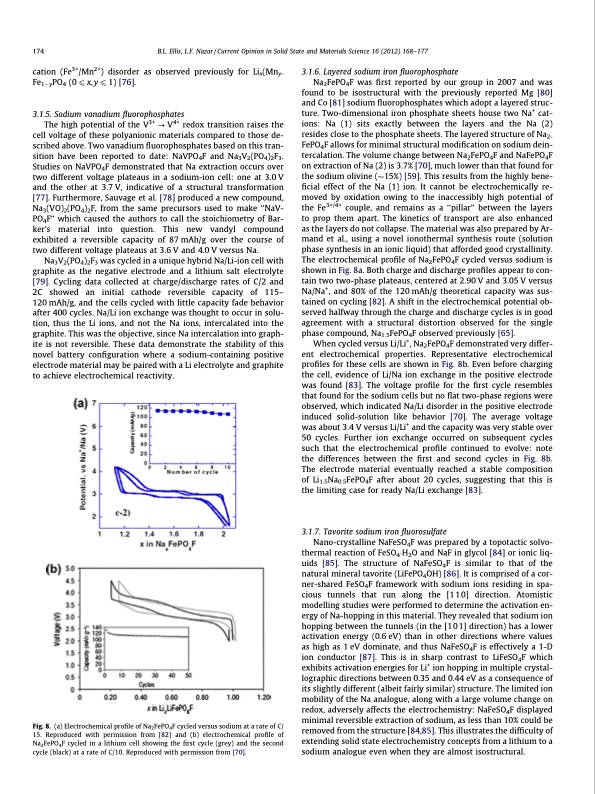
174 B.L. Ellis, L.F. Nazar / Current Opinion in Solid State and Materials Science 16 (2012) 168–177 cation (Fe3+/Mn2+) disorder as observed previously for Lix(Mny- Fe1yPO4 (0 6 x, y 6 1) [76]. 3.1.5. Sodium vanadium fluorophosphates The high potential of the V3+ ? V4+ redox transition raises the cell voltage of these polyanionic materials compared to those de- scribed above. Two vanadium fluorophosphates based on this tran- sition have been reported to date: NaVPO4F and Na3V2(PO4)2F3. Studies on NaVPO4F demonstrated that Na extraction occurs over two different voltage plateaus in a sodium-ion cell: one at 3.0 V and the other at 3.7 V, indicative of a structural transformation [77]. Furthermore, Sauvage et al. [78] produced a new compound, Na3(VO)2(PO4)2F, from the same precursors used to make ‘‘NaV- PO4F’’ which caused the authors to call the stoichiometry of Bar- ker’s material into question. This new vandyl compound exhibited a reversible capacity of 87 mAh/g over the course of two different voltage plateaus at 3.6 V and 4.0 V versus Na. Na3V2(PO4)2F3 was cycled in a unique hybrid Na/Li-ion cell with graphite as the negative electrode and a lithium salt electrolyte [79]. Cycling data collected at charge/discharge rates of C/2 and 2C showed an initial cathode reversible capacity of 115– 120 mAh/g, and the cells cycled with little capacity fade behavior after 400 cycles. Na/Li ion exchange was thought to occur in solu- tion, thus the Li ions, and not the Na ions, intercalated into the graphite. This was the objective, since Na intercalation into graph- ite is not reversible. These data demonstrate the stability of this novel battery configuration where a sodium-containing positive electrode material may be paired with a Li electrolyte and graphite to achieve electrochemical reactivity. 3.1.6. Layered sodium iron fluorophosphate Na2FePO4F was first reported by our group in 2007 and was found to be isostructural with the previously reported Mg [80] and Co [81] sodium fluorophosphates which adopt a layered struc- ture. Two-dimensional iron phosphate sheets house two Na+ cat- ions: Na (1) sits exactly between the layers and the Na (2) resides close to the phosphate sheets. The layered structure of Na2- FePO4F allows for minimal structural modification on sodium dein- tercalation. The volume change between Na2FePO4F and NaFePO4F on extraction of Na (2) is 3.7% [70], much lower than that found for the sodium olivine (15%) [59]. This results from the highly bene- ficial effect of the Na (1) ion. It cannot be electrochemically re- moved by oxidation owing to the inaccessibly high potential of the Fe3+/4+ couple, and remains as a ‘‘pillar’’ between the layers to prop them apart. The kinetics of transport are also enhanced as the layers do not collapse. The material was also prepared by Ar- mand et al., using a novel ionothermal synthesis route (solution phase synthesis in an ionic liquid) that afforded good crystallinity. The electrochemical profile of Na2FePO4F cycled versus sodium is shown in Fig. 8a. Both charge and discharge profiles appear to con- tain two two-phase plateaus, centered at 2.90 V and 3.05 V versus Na/Na+, and 80% of the 120 mAh/g theoretical capacity was sus- tained on cycling [82]. A shift in the electrochemical potential ob- served halfway through the charge and discharge cycles is in good agreement with a structural distortion observed for the single phase compound, Na1.5FePO4F observed previously [65]. When cycled versus Li/Li+, Na2FePO4F demonstrated very differ- ent electrochemical properties. Representative electrochemical profiles for these cells are shown in Fig. 8b. Even before charging the cell, evidence of Li/Na ion exchange in the positive electrode was found [83]. The voltage profile for the first cycle resembles that found for the sodium cells but no flat two-phase regions were observed, which indicated Na/Li disorder in the positive electrode induced solid-solution like behavior [70]. The average voltage was about 3.4 V versus Li/Li+ and the capacity was very stable over 50 cycles. Further ion exchange occurred on subsequent cycles such that the electrochemical profile continued to evolve: note the differences between the first and second cycles in Fig. 8b. The electrode material eventually reached a stable composition of Li1.5Na0.5FePO4F after about 20 cycles, suggesting that this is the limiting case for ready Na/Li exchange [83]. 3.1.7. Tavorite sodium iron fluorosulfate Nano-crystalline NaFeSO4F was prepared by a topotactic solvo- thermal reaction of FeSO4H2O and NaF in glycol [84] or ionic liq- uids [85]. The structure of NaFeSO4F is similar to that of the natural mineral tavorite (LiFePO4OH) [86]. It is comprised of a cor- ner-shared FeSO4F framework with sodium ions residing in spa- cious tunnels that run along the [1 1 0] direction. Atomistic modelling studies were performed to determine the activation en- ergy of Na-hopping in this material. They revealed that sodium ion hopping between the tunnels (in the [101] direction) has a lower activation energy (0.6 eV) than in other directions where values as high as 1 eV dominate, and thus NaFeSO4F is effectively a 1-D ion conductor [87]. This is in sharp contrast to LiFeSO4F which exhibits activation energies for Li+ ion hopping in multiple crystal- lographic directions between 0.35 and 0.44 eV as a consequence of its slightly different (albeit fairly similar) structure. The limited ion mobility of the Na analogue, along with a large volume change on redox, adversely affects the electrochemistry: NaFeSO4F displayed minimal reversible extraction of sodium, as less than 10% could be removed from the structure [84,85]. This illustrates the difficulty of extending solid state electrochemistry concepts from a lithium to a sodium analogue even when they are almost isostructural. Fig. 8. (a) Electrochemical profile of Na2FePO4F cycled versus sodium at a rate of C/ 15. Reproduced with permission from [82] and (b) electrochemical profile of Na2FePO4F cycled in a lithium cell showing the first cycle (grey) and the second cycle (black) at a rate of C/10. Reproduced with permission from [70].PDF Image | Sodium and sodium-ion energy storage batteries
PDF Search Title:
Sodium and sodium-ion energy storage batteriesOriginal File Name Searched:
2012_Na-battery_review.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |