
PDF Publication Title:
Text from PDF Page: 006
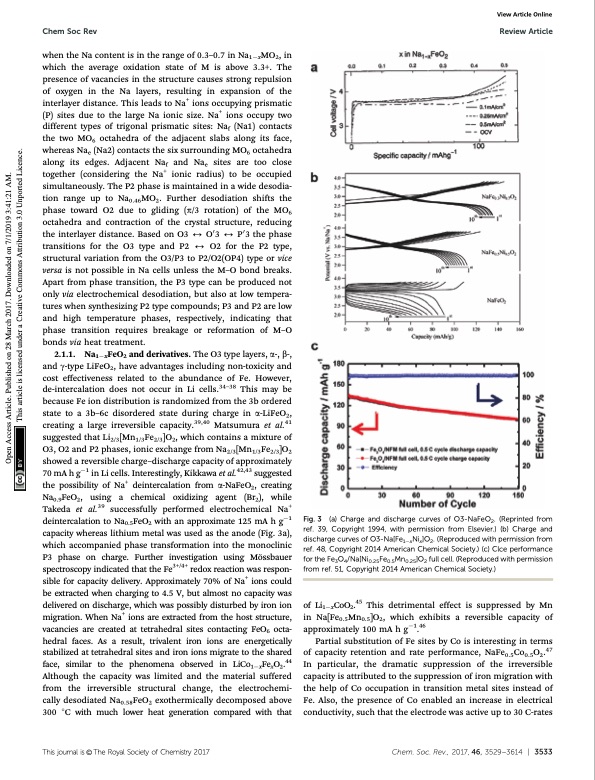
Chem Soc Rev Review Article View Article Online when the Na content is in the range of 0.3–0.7 in Na1xMO2, in which the average oxidation state of M is above 3.3+. The presence of vacancies in the structure causes strong repulsion of oxygen in the Na layers, resulting in expansion of the interlayer distance. This leads to Na+ ions occupying prismatic (P) sites due to the large Na ionic size. Na+ ions occupy two different types of trigonal prismatic sites: Naf (Na1) contacts the two MO6 octahedra of the adjacent slabs along its face, whereas Nae (Na2) contacts the six surrounding MO6 octahedra along its edges. Adjacent Naf and Nae sites are too close together (considering the Na+ ionic radius) to be occupied simultaneously. The P2 phase is maintained in a wide desodia- tion range up to Na0.46MO2. Further desodiation shifts the phase toward O2 due to gliding (p/3 rotation) of the MO6 octahedra and contraction of the crystal structure, reducing the interlayer distance. Based on O3 2 O03 2 P03 the phase transitions for the O3 type and P2 2 O2 for the P2 type, structural variation from the O3/P3 to P2/O2(OP4) type or vice versa is not possible in Na cells unless the M–O bond breaks. Apart from phase transition, the P3 type can be produced not only via electrochemical desodiation, but also at low tempera- tures when synthesizing P2 type compounds; P3 and P2 are low and high temperature phases, respectively, indicating that phase transition requires breakage or reformation of M–O bonds via heat treatment. 2.1.1. Na1xFeO2 and derivatives. The O3 type layers, a-, b-, and g-type LiFeO2, have advantages including non-toxicity and cost effectiveness related to the abundance of Fe. However, de-intercalation does not occur in Li cells.34–38 This may be because Fe ion distribution is randomized from the 3b ordered state to a 3b–6c disordered state during charge in a-LiFeO2, creating a large irreversible capacity.39,40 Matsumura et al.41 suggested that Li2/3[Mn1/3Fe2/3]O2, which contains a mixture of O3, O2 and P2 phases, ionic exchange from Na2/3[Mn1/3Fe2/3]O2 showed a reversible charge–discharge capacity of approximately 70 mA h g1 in Li cells. Interestingly, Kikkawa et al.42,43 suggested the possibility of Na+ deintercalation from a-NaFeO2, creating Na0.9FeO2, using a chemical oxidizing agent (Br2), while Takeda et al.39 successfully performed electrochemical Na+ deintercalation to Na0.5FeO2 with an approximate 125 mA h g1 capacity whereas lithium metal was used as the anode (Fig. 3a), which accompanied phase transformation into the monoclinic P3 phase on charge. Further investigation using Mo ̈ssbauer spectroscopy indicated that the Fe3+/4+ redox reaction was respon- sible for capacity delivery. Approximately 70% of Na+ ions could be extracted when charging to 4.5 V, but almost no capacity was delivered on discharge, which was possibly disturbed by iron ion migration. When Na+ ions are extracted from the host structure, vacancies are created at tetrahedral sites contacting FeO6 octa- hedral faces. As a result, trivalent iron ions are energetically stabilized at tetrahedral sites and iron ions migrate to the shared face, similar to the phenomena observed in LiCo1xFexO2.44 Although the capacity was limited and the material suffered from the irreversible structural change, the electrochemi- cally desodiated Na0.58FeO2 exothermically decomposed above 300 1C with much lower heat generation compared with that Fig. 3 of Li1xCoO2.45 This detrimental effect is suppressed by Mn in Na[Fe0.5Mn0.5]O2, which exhibits a reversible capacity of approximately 100 mA h g1.46 Partial substitution of Fe sites by Co is interesting in terms of capacity retention and rate performance, NaFe0.5Co0.5O2.47 In particular, the dramatic suppression of the irreversible capacity is attributed to the suppression of iron migration with the help of Co occupation in transition metal sites instead of Fe. Also, the presence of Co enabled an increase in electrical conductivity, such that the electrode was active up to 30 C-rates (a) Charge and discharge curves of O3-NaFeO2. (Reprinted from ref. 39, Copyright 1994, with permission from Elsevier.) (b) Charge and discharge curves of O3-Na[Fe1xNix]O2. (Reproduced with permission from ref. 48, Copyright 2014 American Chemical Society.) (c) Clce performance for the Fe3O4/Na[Ni0.25Fe0.5Mn0.25]O2 full cell. (Reproduced with permission from ref. 51, Copyright 2014 American Chemical Society.) Thisjournalis©TheRoyalSocietyofChemistry2017 Chem.Soc.Rev.,2017,46,3529--3614 | 3533 Open Access Article. Published on 28 March 2017. Downloaded on 7/1/2019 3:41:21 AM. This article is licensed under a Creative Commons Attribution 3.0 Unported Licence.PDF Image | Sodium-ion batteries present and future
PDF Search Title:
Sodium-ion batteries present and futureOriginal File Name Searched:
Sodium-ion batteries present and future.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |