
PDF Publication Title:
Text from PDF Page: 036
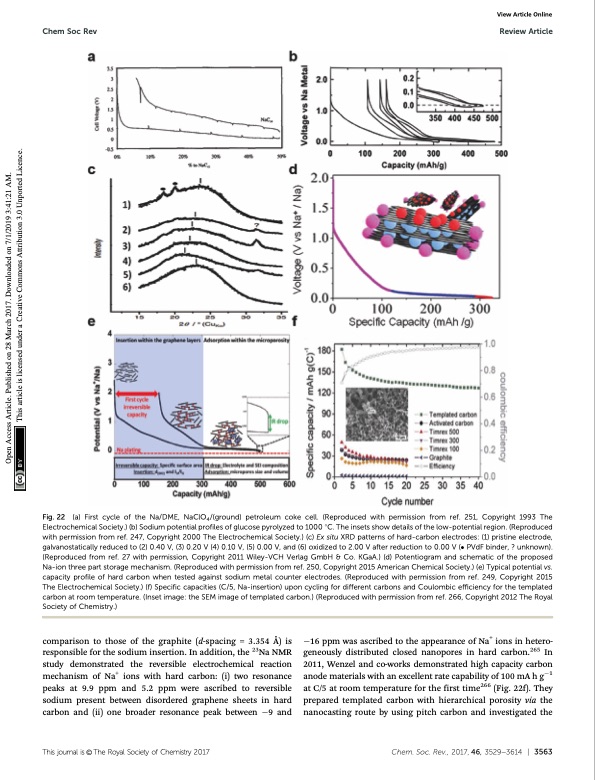
Chem Soc Rev Review Article Fig. 22 (a) First cycle of the Na/DME, NaClO4/(ground) petroleum coke cell. (Reproduced with permission from ref. 251, Copyright 1993 The Electrochemical Society.) (b) Sodium potential profiles of glucose pyrolyzed to 1000 1C. The insets show details of the low-potential region. (Reproduced with permission from ref. 247, Copyright 2000 The Electrochemical Society.) (c) Ex situ XRD patterns of hard-carbon electrodes: (1) pristine electrode, galvanostatically reduced to (2) 0.40 V, (3) 0.20 V (4) 0.10 V, (5) 0.00 V, and (6) oxidized to 2.00 V after reduction to 0.00 V ( PVdF binder, ? unknown). (Reproduced from ref. 27 with permission, Copyright 2011 Wiley-VCH Verlag GmbH & Co. KGaA.) (d) Potentiogram and schematic of the proposed Na-ion three part storage mechanism. (Reproduced with permission from ref. 250, Copyright 2015 American Chemical Society.) (e) Typical potential vs. capacity profile of hard carbon when tested against sodium metal counter electrodes. (Reproduced with permission from ref. 249, Copyright 2015 The Electrochemical Society.) (f) Specific capacities (C/5, Na-insertion) upon cycling for different carbons and Coulombic efficiency for the templated carbon at room temperature. (Inset image: the SEM image of templated carbon.) (Reproduced with permission from ref. 266, Copyright 2012 The Royal Society of Chemistry.) View Article Online comparison to those of the graphite (d-spacing = 3.354 Å) is responsible for the sodium insertion. In addition, the 23Na NMR study demonstrated the reversible electrochemical reaction mechanism of Na+ ions with hard carbon: (i) two resonance peaks at 9.9 ppm and 5.2 ppm were ascribed to reversible sodium present between disordered graphene sheets in hard carbon and (ii) one broader resonance peak between 9 and 16 ppm was ascribed to the appearance of Na+ ions in hetero- geneously distributed closed nanopores in hard carbon.265 In 2011, Wenzel and co-works demonstrated high capacity carbon anode materials with an excellent rate capability of 100 mA h g1 at C/5 at room temperature for the first time266 (Fig. 22f). They prepared templated carbon with hierarchical porosity via the nanocasting route by using pitch carbon and investigated the Thisjournalis©TheRoyalSocietyofChemistry2017 Chem.Soc.Rev.,2017,46,3529--3614 | 3563 Open Access Article. Published on 28 March 2017. Downloaded on 7/1/2019 3:41:21 AM. This article is licensed under a Creative Commons Attribution 3.0 Unported Licence.PDF Image | Sodium-ion batteries present and future
PDF Search Title:
Sodium-ion batteries present and futureOriginal File Name Searched:
Sodium-ion batteries present and future.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |