
PDF Publication Title:
Text from PDF Page: 044
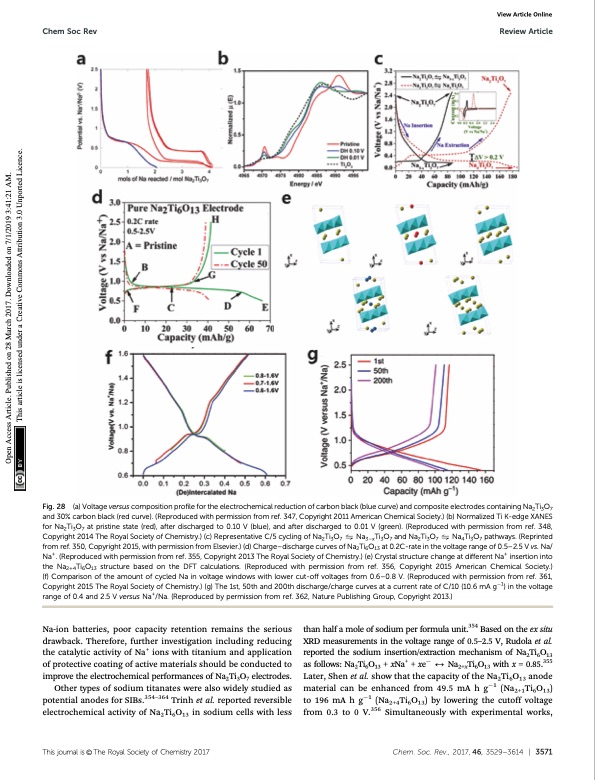
Chem Soc Rev Review Article Fig. 28 (a) Voltage versus composition profile for the electrochemical reduction of carbon black (blue curve) and composite electrodes containing Na2Ti3O7 and 30% carbon black (red curve). (Reproduced with permission from ref. 347, Copyright 2011 American Chemical Society.) (b) Normalized Ti K-edge XANES for Na2Ti3O7 at pristine state (red), after discharged to 0.10 V (blue), and after discharged to 0.01 V (green). (Reproduced with permission from ref. 348, Copyright 2014 The Royal Society of Chemistry.) (c) Representative C/5 cycling of Na2Ti3O7 ! Na3–xTi3O7 and Na2Ti3O7 ! Na4Ti3O7 pathways. (Reprinted from ref. 350, Copyright 2015, with permission from Elsevier.) (d) Charge–discharge curves of Na2Ti6O13 at 0.2C-rate in the voltage range of 0.5–2.5 V vs. Na/ Na+. (Reproduced with permission from ref. 355, Copyright 2013 The Royal Society of Chemistry.) (e) Crystal structure change at different Na+ insertion into the Na2+4Ti6O13 structure based on the DFT calculations. (Reproduced with permission from ref. 356, Copyright 2015 American Chemical Society.) (f) Comparison of the amount of cycled Na in voltage windows with lower cut-off voltages from 0.6–0.8 V. (Reproduced with permission from ref. 361, Copyright 2015 The Royal Society of Chemistry.) (g) The 1st, 50th and 200th discharge/charge curves at a current rate of C/10 (10.6 mA g1) in the voltage range of 0.4 and 2.5 V versus Na+/Na. (Reproduced by permission from ref. 362, Nature Publishing Group, Copyright 2013.) View Article Online Na-ion batteries, poor capacity retention remains the serious drawback. Therefore, further investigation including reducing the catalytic activity of Na+ ions with titanium and application of protective coating of active materials should be conducted to improve the electrochemical performances of Na2Ti3O7 electrodes. Other types of sodium titanates were also widely studied as potential anodes for SIBs.354–364 Trinh et al. reported reversible electrochemical activity of Na2Ti6O13 in sodium cells with less than half a mole of sodium per formula unit.354 Based on the ex situ XRD measurements in the voltage range of 0.5–2.5 V, Rudola et al. reported the sodium insertion/extraction mechanism of Na2Ti6O13 as follows: Na2Ti6O13 + xNa+ + xe 2 Na2+xTi6O13 with x = 0.85.355 Later, Shen et al. show that the capacity of the Na2Ti6O13 anode material can be enhanced from 49.5 mA h g1 (Na2+1Ti6O13) to 196 mA h g1 (Na2+4Ti6O13) by lowering the cutoff voltage from 0.3 to 0 V.356 Simultaneously with experimental works, Thisjournalis©TheRoyalSocietyofChemistry2017 Chem.Soc.Rev.,2017,46,3529--3614 | 3571 Open Access Article. Published on 28 March 2017. Downloaded on 7/1/2019 3:41:21 AM. This article is licensed under a Creative Commons Attribution 3.0 Unported Licence.PDF Image | Sodium-ion batteries present and future
PDF Search Title:
Sodium-ion batteries present and futureOriginal File Name Searched:
Sodium-ion batteries present and future.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |