
PDF Publication Title:
Text from PDF Page: 046
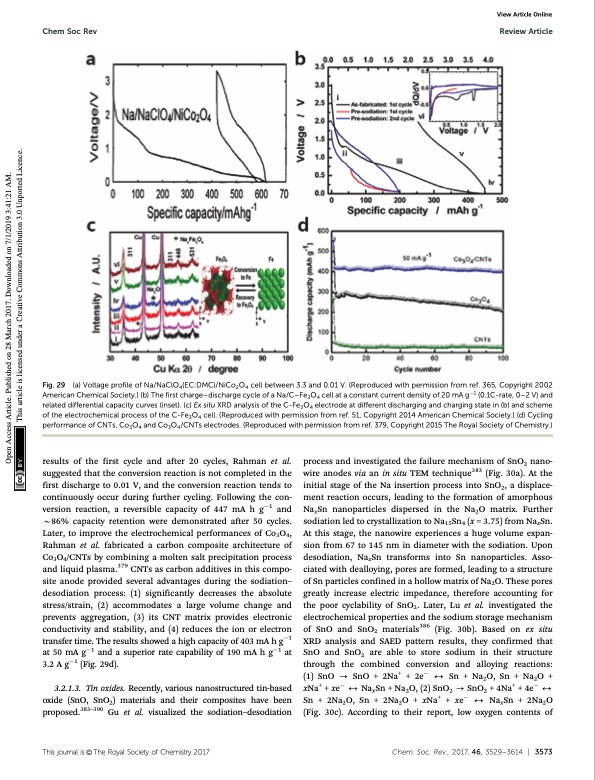
Chem Soc Rev Review Article Fig. 29 (a) Voltage profile of Na/NaClO4(EC:DMC)/NiCo2O4 cell between 3.3 and 0.01 V. (Reproduced with permission from ref. 365, Copyright 2002 American Chemical Society.) (b) The first charge–discharge cycle of a Na/C–Fe3O4 cell at a constant current density of 20 mA g1 (0.1C-rate, 0–2 V) and related differential capacity curves (inset). (c) Ex situ XRD analysis of the C-Fe3O4 electrode at different discharging and charging state in (b) and scheme of the electrochemical process of the C-Fe3O4 cell. (Reproduced with permission from ref. 51, Copyright 2014 American Chemical Society.) (d) Cycling performance of CNTs, Co3O4 and Co3O4/CNTs electrodes. (Reproduced with permission from ref. 379, Copyright 2015 The Royal Society of Chemistry.) View Article Online results of the first cycle and after 20 cycles, Rahman et al. suggested that the conversion reaction is not completed in the first discharge to 0.01 V, and the conversion reaction tends to continuously occur during further cycling. Following the con- version reaction, a reversible capacity of 447 mA h g1 and B86% capacity retention were demonstrated after 50 cycles. Later, to improve the electrochemical performances of Co3O4, Rahman et al. fabricated a carbon composite architecture of Co3O4/CNTs by combining a molten salt precipitation process and liquid plasma.379 CNTs as carbon additives in this compo- site anode provided several advantages during the sodiation– desodiation process: (1) significantly decreases the absolute stress/strain, (2) accommodates a large volume change and prevents aggregation, (3) its CNT matrix provides electronic conductivity and stability, and (4) reduces the ion or electron transfer time. The results showed a high capacity of 403 mA h g1 at 50 mA g1 and a superior rate capability of 190 mA h g1 at 3.2 A g1 (Fig. 29d). 3.2.1.3. Tin oxides. Recently, various nanostructured tin-based oxide (SnO, SnO2) materials and their composites have been proposed.383–390 Gu et al. visualized the sodiation–desodiation process and investigated the failure mechanism of SnO2 nano- wire anodes via an in situ TEM technique383 (Fig. 30a). At the initial stage of the Na insertion process into SnO2, a displace- ment reaction occurs, leading to the formation of amorphous NaxSn nanoparticles dispersed in the Na2O matrix. Further sodiation led to crystallization to Na15Sn4 (x = 3.75) from NaxSn. At this stage, the nanowire experiences a huge volume expan- sion from 67 to 145 nm in diameter with the sodiation. Upon desodiation, NaxSn transforms into Sn nanoparticles. Asso- ciated with dealloying, pores are formed, leading to a structure of Sn particles confined in a hollow matrix of Na2O. These pores greatly increase electric impedance, therefore accounting for the poor cyclability of SnO2. Later, Lu et al. investigated the electrochemical properties and the sodium storage mechanism of SnO and SnO2 materials386 (Fig. 30b). Based on ex situ XRD analysis and SAED pattern results, they confirmed that SnO and SnO2 are able to store sodium in their structure through the combined conversion and alloying reactions: (1)SnO-SnO+2Na+ +2e 2Sn+Na2O,Sn+Na2O+ xNa+ +xe 2NaxSn+Na2O,(2)SnO2 -SnO2 +4Na+ +4e 2 Sn+2Na2O,Sn+2Na2O+xNa+ +xe 2NaxSn+2Na2O (Fig. 30c). According to their report, low oxygen contents of Thisjournalis©TheRoyalSocietyofChemistry2017 Chem.Soc.Rev.,2017,46,3529--3614 | 3573 Open Access Article. Published on 28 March 2017. Downloaded on 7/1/2019 3:41:21 AM. This article is licensed under a Creative Commons Attribution 3.0 Unported Licence.PDF Image | Sodium-ion batteries present and future
PDF Search Title:
Sodium-ion batteries present and futureOriginal File Name Searched:
Sodium-ion batteries present and future.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |