
PDF Publication Title:
Text from PDF Page: 053
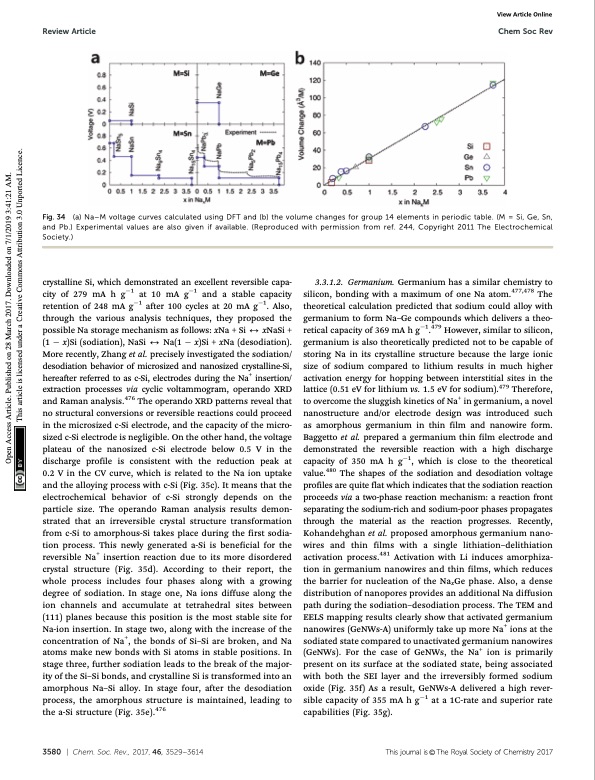
Review Article Chem Soc Rev Fig. 34 (a) Na–M voltage curves calculated using DFT and (b) the volume changes for group 14 elements in periodic table. (M = Si, Ge, Sn, and Pb.) Experimental values are also given if available. (Reproduced with permission from ref. 244, Copyright 2011 The Electrochemical Society.) View Article Online crystalline Si, which demonstrated an excellent reversible capa- city of 279 mA h g1 at 10 mA g1 and a stable capacity retention of 248 mA g1 after 100 cycles at 20 mA g1. Also, through the various analysis techniques, they proposed the possible Na storage mechanism as follows: xNa + Si 2 xNaSi + (1 x)Si (sodiation), NaSi 2 Na(1 x)Si + xNa (desodiation). More recently, Zhang et al. precisely investigated the sodiation/ desodiation behavior of microsized and nanosized crystalline-Si, hereafter referred to as c-Si, electrodes during the Na+ insertion/ extraction processes via cyclic voltammogram, operando XRD and Raman analysis.476 The operando XRD patterns reveal that no structural conversions or reversible reactions could proceed in the microsized c-Si electrode, and the capacity of the micro- sized c-Si electrode is negligible. On the other hand, the voltage plateau of the nanosized c-Si electrode below 0.5 V in the discharge profile is consistent with the reduction peak at 0.2 V in the CV curve, which is related to the Na ion uptake and the alloying process with c-Si (Fig. 35c). It means that the electrochemical behavior of c-Si strongly depends on the particle size. The operando Raman analysis results demon- strated that an irreversible crystal structure transformation from c-Si to amorphous-Si takes place during the first sodia- tion process. This newly generated a-Si is beneficial for the reversible Na+ insertion reaction due to its more disordered crystal structure (Fig. 35d). According to their report, the whole process includes four phases along with a growing degree of sodiation. In stage one, Na ions diffuse along the ion channels and accumulate at tetrahedral sites between (111) planes because this position is the most stable site for Na-ion insertion. In stage two, along with the increase of the concentration of Na+, the bonds of Si–Si are broken, and Na atoms make new bonds with Si atoms in stable positions. In stage three, further sodiation leads to the break of the major- ity of the Si–Si bonds, and crystalline Si is transformed into an amorphous Na–Si alloy. In stage four, after the desodiation process, the amorphous structure is maintained, leading to the a-Si structure (Fig. 35e).476 3.3.1.2. Germanium. Germanium has a similar chemistry to silicon, bonding with a maximum of one Na atom.477,478 The theoretical calculation predicted that sodium could alloy with germanium to form Na–Ge compounds which delivers a theo- retical capacity of 369 mA h g1.479 However, similar to silicon, germanium is also theoretically predicted not to be capable of storing Na in its crystalline structure because the large ionic size of sodium compared to lithium results in much higher activation energy for hopping between interstitial sites in the lattice (0.51 eV for lithium vs. 1.5 eV for sodium).479 Therefore, to overcome the sluggish kinetics of Na+ in germanium, a novel nanostructure and/or electrode design was introduced such as amorphous germanium in thin film and nanowire form. Baggetto et al. prepared a germanium thin film electrode and demonstrated the reversible reaction with a high discharge capacity of 350 mA h g1, which is close to the theoretical value.480 The shapes of the sodiation and desodiation voltage profiles are quite flat which indicates that the sodiation reaction proceeds via a two-phase reaction mechanism: a reaction front separating the sodium-rich and sodium-poor phases propagates through the material as the reaction progresses. Recently, Kohandehghan et al. proposed amorphous germanium nano- wires and thin films with a single lithiation–delithiation activation process.481 Activation with Li induces amorphiza- tion in germanium nanowires and thin films, which reduces the barrier for nucleation of the NaxGe phase. Also, a dense distribution of nanopores provides an additional Na diffusion path during the sodiation–desodiation process. The TEM and EELS mapping results clearly show that activated germanium nanowires (GeNWs-A) uniformly take up more Na+ ions at the sodiated state compared to unactivated germanium nanowires (GeNWs). For the case of GeNWs, the Na+ ion is primarily present on its surface at the sodiated state, being associated with both the SEI layer and the irreversibly formed sodium oxide (Fig. 35f) As a result, GeNWs-A delivered a high rever- sible capacity of 355 mA h g1 at a 1C-rate and superior rate capabilities (Fig. 35g). 3580 | Chem. Soc. Rev., 2017, 46, 3529--3614 This journal is © The Royal Society of Chemistry 2017 Open Access Article. Published on 28 March 2017. Downloaded on 7/1/2019 3:41:21 AM. This article is licensed under a Creative Commons Attribution 3.0 Unported Licence.PDF Image | Sodium-ion batteries present and future
PDF Search Title:
Sodium-ion batteries present and futureOriginal File Name Searched:
Sodium-ion batteries present and future.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |