
PDF Publication Title:
Text from PDF Page: 068
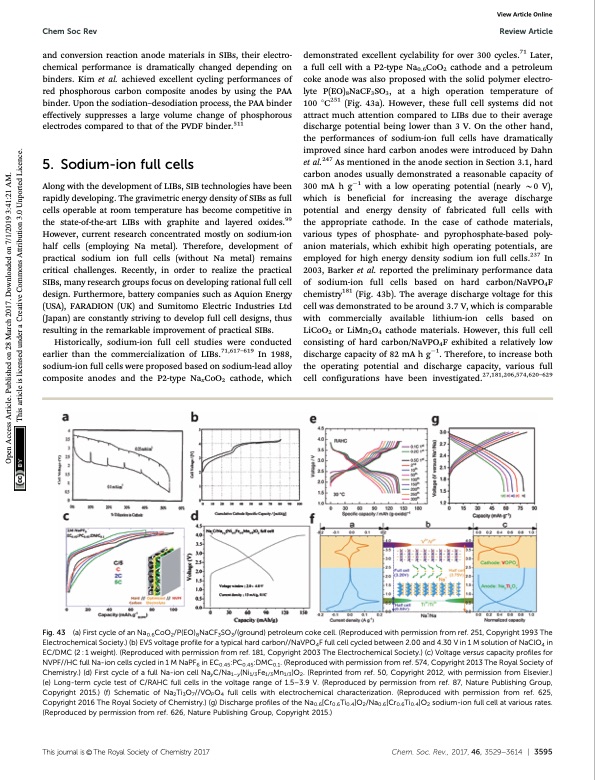
Chem Soc Rev Review Article demonstrated excellent cyclability for over 300 cycles.71 Later, a full cell with a P2-type Na0.6CoO2 cathode and a petroleum coke anode was also proposed with the solid polymer electro- lyte P(EO)8NaCF3SO3, at a high operation temperature of 100 1C251 (Fig. 43a). However, these full cell systems did not attract much attention compared to LIBs due to their average discharge potential being lower than 3 V. On the other hand, the performances of sodium-ion full cells have dramatically improved since hard carbon anodes were introduced by Dahn et al.247 As mentioned in the anode section in Section 3.1, hard carbon anodes usually demonstrated a reasonable capacity of 300 mA h g1 with a low operating potential (nearly B0 V), which is beneficial for increasing the average discharge potential and energy density of fabricated full cells with the appropriate cathode. In the case of cathode materials, various types of phosphate- and pyrophosphate-based poly- anion materials, which exhibit high operating potentials, are employed for high energy density sodium ion full cells.237 In 2003, Barker et al. reported the preliminary performance data of sodium-ion full cells based on hard carbon/NaVPO4F chemistry181 (Fig. 43b). The average discharge voltage for this cell was demonstrated to be around 3.7 V, which is comparable with commercially available lithium-ion cells based on LiCoO2 or LiMn2O4 cathode materials. However, this full cell consisting of hard carbon/NaVPO4F exhibited a relatively low discharge capacity of 82 mA h g1. Therefore, to increase both the operating potential and discharge capacity, various full cell configurations have been investigated.27,181,206,574,620–629 and conversion reaction anode materials in SIBs, their electro- chemical performance is dramatically changed depending on binders. Kim et al. achieved excellent cycling performances of red phosphorous carbon composite anodes by using the PAA binder. Upon the sodiation–desodiation process, the PAA binder effectively suppresses a large volume change of phosphorous electrodes compared to that of the PVDF binder.511 5. Sodium-ion full cells Along with the development of LIBs, SIB technologies have been rapidly developing. The gravimetric energy density of SIBs as full cells operable at room temperature has become competitive in the state-of-the-art LIBs with graphite and layered oxides.99 However, current research concentrated mostly on sodium-ion half cells (employing Na metal). Therefore, development of practical sodium ion full cells (without Na metal) remains critical challenges. Recently, in order to realize the practical SIBs, many research groups focus on developing rational full cell design. Furthermore, battery companies such as Aquion Energy (USA), FARADION (UK) and Sumitomo Electric Industries Ltd (Japan) are constantly striving to develop full cell designs, thus resulting in the remarkable improvement of practical SIBs. Historically, sodium-ion full cell studies were conducted earlier than the commercialization of LIBs.71,617–619 In 1988, sodium-ion full cells were proposed based on sodium-lead alloy composite anodes and the P2-type NaxCoO2 cathode, which View Article Online Fig. 43 (a) First cycle of an Na0.6CoO2/P(EO)8NaCF3SO3/(ground) petroleum coke cell. (Reproduced with permission from ref. 251, Copyright 1993 The Electrochemical Society.) (b) EVS voltage profile for a typical hard carbon//NaVPO4F full cell cycled between 2.00 and 4.30 V in 1 M solution of NaClO4 in EC/DMC (2 : 1 weight). (Reproduced with permission from ref. 181, Copyright 2003 The Electrochemical Society.) (c) Voltage versus capacity profiles for NVPF//HC full Na-ion cells cycled in 1 M NaPF6 in EC0.45:PC0.45:DMC0.1. (Reproduced with permission from ref. 574, Copyright 2013 The Royal Society of Chemistry.) (d) First cycle of a full Na-ion cell NayC/Na1–y(Ni1/3Fe1/3Mn1/3)O2. (Reprinted from ref. 50, Copyright 2012, with permission from Elsevier.) (e) Long-term cycle test of C/RAHC full cells in the voltage range of 1.5–3.9 V. (Reproduced by permission from ref. 87, Nature Publishing Group, Copyright 2015.) (f) Schematic of Na2Ti3O7//VOPO4 full cells with electrochemical characterization. (Reproduced with permission from ref. 625, Copyright 2016 The Royal Society of Chemistry.) (g) Discharge profiles of the Na0.6[Cr0.6Ti0.4]O2/Na0.6[Cr0.6Ti0.4]O2 sodium-ion full cell at various rates. (Reproduced by permission from ref. 626, Nature Publishing Group, Copyright 2015.) Thisjournalis©TheRoyalSocietyofChemistry2017 Chem.Soc.Rev.,2017,46,3529--3614 | 3595 Open Access Article. Published on 28 March 2017. Downloaded on 7/1/2019 3:41:21 AM. This article is licensed under a Creative Commons Attribution 3.0 Unported Licence.PDF Image | Sodium-ion batteries present and future
PDF Search Title:
Sodium-ion batteries present and futureOriginal File Name Searched:
Sodium-ion batteries present and future.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |