
PDF Publication Title:
Text from PDF Page: 072
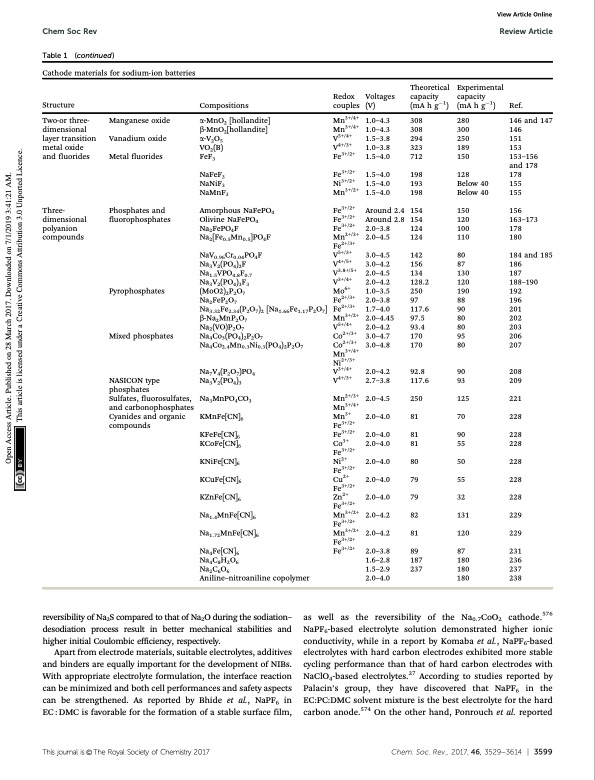
Chem Soc Rev Review Article Experimental capacity (mA h g1) Ref. Table 1 (continued) Cathode materials for sodium-ion batteries Manganese oxide Vanadium oxide Metal fluorides Phosphates and fluorophosphates Pyrophosphates Mixed phosphates NASICON type phosphates Sulfates, fluorosulfates, and carbonophosphates Cyanides and organic compounds 146 and 147 View Article Online Structure Two-or three- dimensional layer transition metal oxide and fluorides Three- dimensional polyanion compounds Compositions a-MnO2 [hollandite] b-MnO2[hollandite] a-V2O5 VO2(B) FeF3 NaFeF3 NaNiF3 NaMnF3 Amorphous NaFePO4 Olivine NaFePO4 Na2FePO4F Na2[Fe0.5Mn0.5]PO4F NaV0.96Cr0.04PO4F Na3V2(PO4)2F Na1.5VPO4.8F0.7 Na3V2(PO4)3F3 (MoO2)2P2O7 Na2FeP2O7 Na3.32Fe2.34(P2O7)2 [Na1.66Fe1.17P2O7] b-Na2MnP2O7 Na2(VO)P2O7 Na4Co3(PO4)2P2O7 Na4Co2.4Mn0.3Ni0.3(PO4)2P2O7 Na7V4(P2O7)PO4 Na3V2(PO4)3 Na3MnPO4CO3 KMnFe[CN]6 KFeFe[CN]6 KCoFe[CN]6 KNiFe[CN]6 KCuFe[CN]6 KZnFe[CN]6 Na1.4MnFe[CN]6 Na1.72MnFe[CN]6 Na4Fe[CN]6 Na4C8H2O6 Na2C6O6 Aniline–nitroaniline copolymer Redox Voltages couples (V) Mn3+/4+ 1.0–4.3 Mn3+/4+ 1.0–4.3 V5+/4+ 1.5–3.8 V4+/3+ 1.0–3.8 Fe3+/2+ 1.5–4.0 Fe3+/2+ 1.5–4.0 Ni3+/2+ 1.5–4.0 Mn3+/2+ 1.5–4.0 Fe3+/2+ Around 2.4 Fe3+/2+ Around 2.8 Fe3+/2+ 2.0–3.8 Mn2+/3+ 2.0–4.5 Fe2+/3+ V5+/3+ 3.0–4.5 V4+/5+ 3.0–4.2 V3.8+/5+ 2.0–4.5 V3+/4+ 2.0–4.2 Mo6+ 1.0–3.5 Fe2+/3+ 2.0–3.8 Fe2+/3+ 1.7–4.0 Mn3+/2+ 2.0–4.45 V5+/4+ 2.0–4.2 Co2+/3+ 3.0–4.7 Co2+/3+ 3.0–4.8 Mn3+/4+ Ni2+/3+ V3+/4+ 2.0–4.2 V4+/3+ 2.7–3.8 Mn2+/3+ 2.0–4.5 Mn3+/4+ Mn3+ 2.0–4.0 Fe3+/2+ Fe3+/2+ 2.0–4.0 Co3+ 2.0–4.0 Fe3+/2+ Ni2+ 2.0–4.0 Fe3+/2+ Cu2+ 2.0–4.0 Fe3+/2+ Zn2+ 2.0–4.0 Fe3+/2+ Mn3+/2+ 2.0–4.2 Fe3+/2+ Mn3+/2+ 2.0–4.2 Fe3+/2+ Fe3+/2+ 2.0–3.8 1.6–2.8 1.5–2.9 2.0–4.0 Theoretical capacity (mA h g1) 308 308 294 323 712 198 193 198 154 154 124 124 142 156 134 128.2 250 97 117.6 97.5 93.4 170 170 92.8 117.6 250 81 81 81 80 79 79 82 81 89 187 237 280 300 146 250 151 189 153 150 153–156 and 178 128 178 Below 40 155 Below 40 155 150 156 120 163–173 100 178 110 180 80 87 186 130 187 120 188–190 190 192 88 196 90 201 80 202 80 203 95 206 80 207 90 208 93 209 125 221 70 228 90 228 55 228 50 228 55 228 32 228 131 229 120 229 87 231 180 236 180 237 180 238 184 and 185 reversibility of Na2S compared to that of Na2O during the sodiation– desodiation process result in better mechanical stabilities and higher initial Coulombic efficiency, respectively. Apart from electrode materials, suitable electrolytes, additives and binders are equally important for the development of NIBs. With appropriate electrolyte formulation, the interface reaction can be minimized and both cell performances and safety aspects can be strengthened. As reported by Bhide et al., NaPF6 in EC : DMC is favorable for the formation of a stable surface film, as well as the reversibility of the Na0.7CoO2 cathode.576 NaPF6-based electrolyte solution demonstrated higher ionic conductivity, while in a report by Komaba et al., NaPF6-based electrolytes with hard carbon electrodes exhibited more stable cycling performance than that of hard carbon electrodes with NaClO4-based electrolytes.27 According to studies reported by Palacin’s group, they have discovered that NaPF6 in the EC:PC:DMC solvent mixture is the best electrolyte for the hard carbon anode.574 On the other hand, Ponrouch et al. reported Thisjournalis©TheRoyalSocietyofChemistry2017 Chem.Soc.Rev.,2017,46,3529--3614 | 3599 Open Access Article. Published on 28 March 2017. Downloaded on 7/1/2019 3:41:21 AM. This article is licensed under a Creative Commons Attribution 3.0 Unported Licence.PDF Image | Sodium-ion batteries present and future
PDF Search Title:
Sodium-ion batteries present and futureOriginal File Name Searched:
Sodium-ion batteries present and future.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |