
PDF Publication Title:
Text from PDF Page: 007
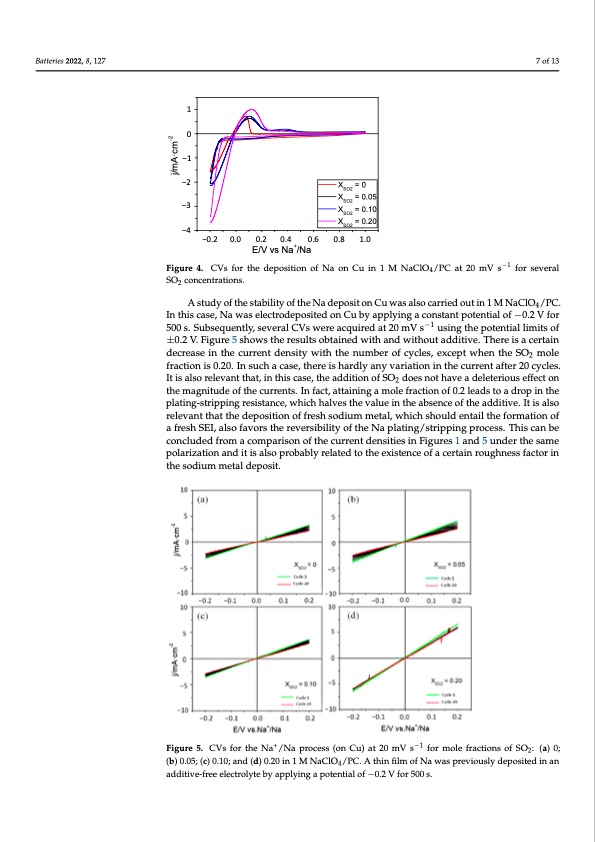
Batteries 2022, 8, x FOR PEER REVIEW Batteries 2022, 8, x FOR PEER REVIEW Batteries 2022, 8, 127 7 of 13 7 of 13 7 of 13 trations. trations. 1 1 0 0 −-1 −-1 −-2 XSO2 = 0 XSO2 = 0.05 X =0 X SO2 = 0.10 0.8 1.0 XSO2 = 0.20 j/mA·cm -2 -2 j/mA·cm −-2 −3 -3 SO2 -3 X = 0.05 X SO2 = 0.20 −3 SO2 −-4 -−0.2 0.0 0.0 0.2 E/V vs Na+/Na XSO2 = 0.10 −-4 -−0.2 0.6 E/V vs Na+/Na SO2 concentrations. 0..4 0.2 0..4 0.6 0.8 1.0 Figure 4. CVs for the deposition of Na on Cu in 1 M NaClO4/PC at 20 mV s −1 for several SO2 concen- −1 Figure 4. CVs for the deposition of Na on Cu in 1 M NaClO4/PC at 20 mV s Figure 4. CVs for the deposition of Na on Cu in 1 M NaClO4/PC at 20 mV s−1 for several SO2 concen- A study of the stability of the Na deposit on Cu was also carried out in 1 M NaClO4/PACs.tIundtyhoisfcthaeses,taNbailiwtyaosfetlhecetNroaddeeppoossitietdonoCnuCwuabsyaalspopclayrinrigedaocuotnisnta1nMt pNotaeCnltOial/PC. A study of the stability of the Na deposit on Cu was also carried out in 1 M −1 of−0I.n2tVhifsocra5s0e0,Ns.aSwubaseeqluecetnrtoldy,espeovseitreadlConVsCwuebryeapcqpulyiirnegdatc2o0nsmtaVntspoutseintgiatlhoefp−o0te.2nV- for NaClO4/PC. In this case, Na was electrodeposited on Cu by applying a constant potential −1 tial l5im00its.oSfu±b0s.e2qVu.eFnitglyu,rsev5esrhaolwCsVtshweereresualctsquoibrteadinaetd20wmithVasnd wusiitnhgouthteadpdoitteivneti.aTlhliemreitsof of −0.2 V for 500 s. Subsequently, several CVs were acquired at 20 mV s−1 using the poten- isac±er0t.a2inV.dFeicgrueraese5isnhtohwescuthrreernetsdueltnssoitbytawinitehdthweitnhuamnbdewroitfhcoyuclteasd,edxictievpet.wThernetihseaScOe2rtain tial limits of ±0.2 V. Figure 5 shows the results obtained with and without additive. There moledefrcarcetaisoeniinst0h.2e0c.uInrrseuncthdaencsaistey,wthiethretihsehnaurdmlybearnyofvcayrcialetiso,nexincetphtewcuhrernenttheafSteOr220mole is a certain decrease in the current density with the number of cycles, except when the SO2 cyclefrsa.cItiiosnaliso0.r2e0le.vInanstutchata,cinasteh,isthcearsee,isthearadldyitainoynvoafrSiOat2iodnoeinsnthoethcuavrrenatdaefltetrer2i0oucyscles. mole fraction is 0.20. In such a case, there is hardly any variation in the current after 20 Itisalsorelevantthat,inthiscase,theadditionofSO doesnothaveadeleteriouseffecton effect on the magnitude of the currents. In fact, attaining2 a mole fraction of 0.2 leads to a cycles. It is also relevant that, in this case, the addition of SO2 does not have a deleterious the magnitude of the currents. In fact, attaining a mole fraction of 0.2 leads to a drop in the drop in the plating-stripping resistance, which halves the value in the absence of the ad- effect on the magnitude of the currents. In fact, attaining a mole fraction of 0.2 leads to a plating-stripping resistance, which halves the value in the absence of the additive. It is also ditive. It is also relevant that the deposition of fresh sodium metal, which should entail drop in the plating-stripping resistance, which halves the value in the absence of the ad- relevant that the deposition of fresh sodium metal, which should entail the formation of the formation of a fresh SEI, also favors the reversibility of the Na plating/stripping pro- ditive. It is also relevant that the deposition of fresh sodium metal, which should entail a fresh SEI, also favors the reversibility of the Na plating/stripping process. This can be cess. This can be concluded from a comparison of the current densities in Figures 1 and 5 the formation of a fresh SEI, also favors the reversibility of the Na plating/stripping pro- concluded from a comparison of the current densities in Figures 1 and 5 under the same under the same polarization and it is also probably related to the existence of a certain cess. This can be concluded from a comparison of the current densities in Figures 1 and 5 polarization and it is also probably related to the existence of a certain roughness factor in roughness factor in the sodium metal deposit. under the same polarization and it is also probably related to the existence of a certain the sodium metal deposit. roughness factor in the sodium metal deposit. Figure 5. CVs for the Na+/Na process (on Cu) at 20 mV s−1 for mole fractions of SO2: (a) 0; (b) 0.05; (c) 0.10; and (d) 0.20 in 1 M NaClO4/PC. A thin film of Na was previously deposited in an additive-free electrolyte by applying a potential of −0.2 V for 500 s. for several 4PDF Image | Sulfur Dioxide and Sulfolane Sodium Batteries
PDF Search Title:
Sulfur Dioxide and Sulfolane Sodium BatteriesOriginal File Name Searched:
batteries-08-00127-v2.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |