
PDF Publication Title:
Text from PDF Page: 003
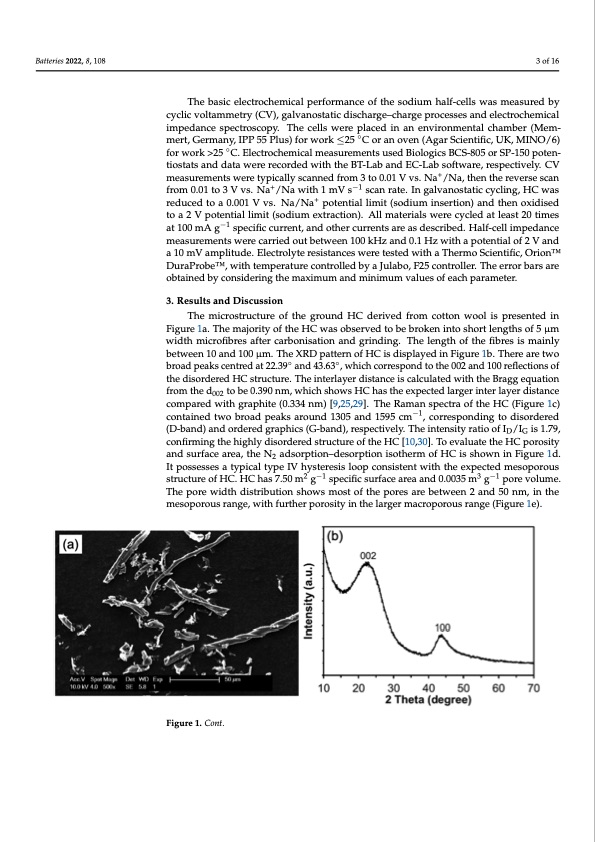
Batteries 2022, 8, 108 3 of 16 The basic electrochemical performance of the sodium half-cells was measured by cyclic voltammetry (CV), galvanostatic discharge–charge processes and electrochemical impedance spectroscopy. The cells were placed in an environmental chamber (Mem- mert, Germany, IPP 55 Plus) for work ≤25 ◦C or an oven (Agar Scientific, UK, MINO/6) for work >25 ◦C. Electrochemical measurements used Biologics BCS-805 or SP-150 poten- tiostats and data were recorded with the BT-Lab and EC-Lab software, respectively. CV measurements were typically scanned from 3 to 0.01 V vs. Na+/Na, then the reverse scan from 0.01 to 3 V vs. Na+/Na with 1 mV s−1 scan rate. In galvanostatic cycling, HC was reduced to a 0.001 V vs. Na/Na+ potential limit (sodium insertion) and then oxidised to a 2 V potential limit (sodium extraction). All materials were cycled at least 20 times at 100 mA g−1 specific current, and other currents are as described. Half-cell impedance measurements were carried out between 100 kHz and 0.1 Hz with a potential of 2 V and a 10 mV amplitude. Electrolyte resistances were tested with a Thermo Scientific, OrionTM DuraProbeTM, with temperature controlled by a Julabo, F25 controller. The error bars are obtained by considering the maximum and minimum values of each parameter. 3. Results and Discussion The microstructure of the ground HC derived from cotton wool is presented in Figure 1a. The majority of the HC was observed to be broken into short lengths of 5 μm width microfibres after carbonisation and grinding. The length of the fibres is mainly between 10 and 100 μm. The XRD pattern of HC is displayed in Figure 1b. There are two broad peaks centred at 22.39◦ and 43.63◦, which correspond to the 002 and 100 reflections of the disordered HC structure. The interlayer distance is calculated with the Bragg equation from the d002 to be 0.390 nm, which shows HC has the expected larger inter layer distance compared with graphite (0.334 nm) [9,25,29]. The Raman spectra of the HC (Figure 1c) contained two broad peaks around 1305 and 1595 cm−1, corresponding to disordered (D-band) and ordered graphics (G-band), respectively. The intensity ratio of ID/IG is 1.79, confirming the highly disordered structure of the HC [10,30]. To evaluate the HC porosity and surface area, the N2 adsorption–desorption isotherm of HC is shown in Figure 1d. It possesses a typical type IV hysteresis loop consistent with the expected mesoporous structure of HC. HC has 7.50 m2 g−1 specific surface area and 0.0035 m3 g−1 pore volume. Batteries 2022, 8, x FOR PEER REVIEW 4 of 16 The pore width distribution shows most of the pores are between 2 and 50 nm, in the mesoporous range, with further porosity in the larger macroporous range (Figure 1e). Figure 1. Cont.PDF Image | Temperature Dependence of Hard Carbon Sodium Half-Cells
PDF Search Title:
Temperature Dependence of Hard Carbon Sodium Half-CellsOriginal File Name Searched:
batteries_08_00108_v2.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |