
PDF Publication Title:
Text from PDF Page: 007
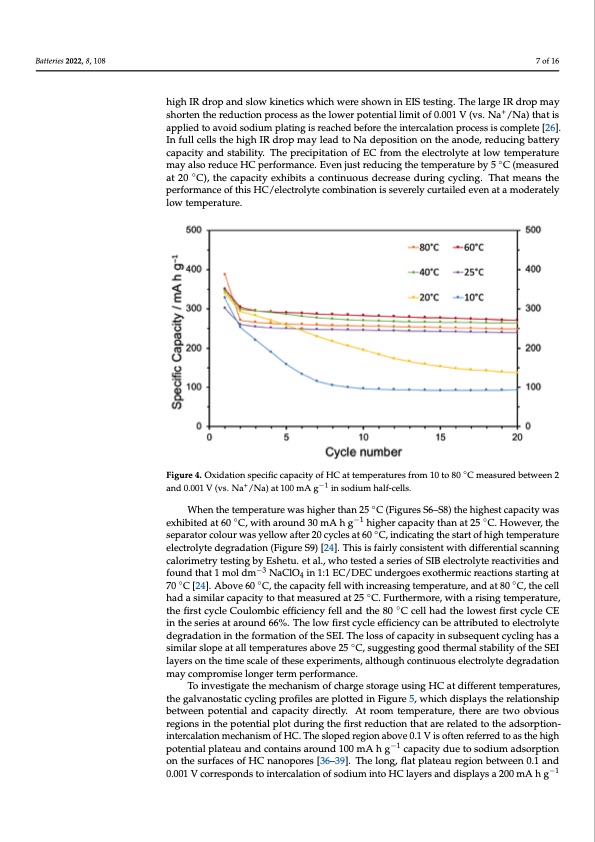
Batteries 2022, 8, 108 tion capacity (Figure S4) reduces by less than 15 mA h g−1 over 20 cycles. The initial Cou- lombic efficiency (oxidation capacity divided by reduction capacity) is 83%, and that rises to 99.3% after 20 cycles (Figure S5). When the temperature is below 25 °C, there is a sig- nificant capacity drop. At 10 or 15 °C, less than 40% of the initial capacity was reta7inoef d16 after 20 cycles (Figures S3–S5). The battery at 10 °C displays a significant drop in capacity over the first few cycles, but becomes stable after 9 cycles. The capacity drops may be due to the high IR drop and slow kinetics which were shown in EIS testing. The large IR drop high IR drop and slow kinetics which were shown in EIS testing. The large IR drop may may shorten the reduction process as the lower potential limit of 0.001 V (vs. N+ a+/Na) that shorten the reduction process as the lower potential limit of 0.001 V (vs. Na /Na) that is is applied to avoid sodium plating is reached before the intercalation process is complete applied to avoid sodium plating is reached before the intercalation process is complete [26]. [26]. In full cells the high IR drop may lead to Na deposition on the anode, reducing bat- In full cells the high IR drop may lead to Na deposition on the anode, reducing battery tery capacity and stability. The precipitation of EC from the electrolyte at low temperature capacity and stability. The precipitation of EC from the electrolyte at low temperature may also reduce HC performance. Even just reducing the temperature by 5 °◦C (measured may also reduce HC performance. Even just reducing the temperature by 5 C (measured at 20 °C◦ ), the capacity exhibits a continuous decrease during cycling. That means the per- at 20 C), the capacity exhibits a continuous decrease during cycling. That means the formance of this HC/electrolyte combination is severely curtailed even at a moderately performance of this HC/electrolyte combination is severely curtailed even at a moderately low temperature. low temperature. Figure 4. Oxidation specific capacity of HC at temperatures from 10 to 80 °C measured between 2 Figure 4. Oxidation specific capacity of HC at temperatures from 10 to 80 C measured between 2 and 0.001 V (vs. Na+/+Na) at 100 mA g−1 −in1 sodium half-cells. and 0.001 V (vs. Na /Na) at 100 mA g in sodium half-cells. ◦ Wheenthetemperaturewaasshhigighheerrthtahnan2525C°C(Fi(gFuigruesreSs6–SS68–)St8h)ethiegheigsthceasptaccaiptyacwitays ◦ −1−1 ◦ wexahsiebxitheidbiatetd60atC60,w°Cit,hwairtohuanrdou3n0dm3A0mhAg hghighiegrhcearpcacpitaycitthyatnhant2a5t2C5.°HC.oHwoewveerv,ethr,e ◦ tshepsaerpataorractorlocuorlowuarswyealsloywellaofwtera2f0tecry2c0lecsyactl6e0s aCt 6, 0in°dCic,aitnindgictahtiensgtatrhteofsthairgthotfehmigphertaetmur-e peeleractruorleyeteledcetrgorlaydteatdioengr(aFdigautiroenS(9F)ig[2u4r]e. TS9h)is[2is4]f.aTirhlyisciosnfasisrtlyenctownsitihstedniftfewreitnhtidailffsecraenntinalg scalnonrimngetcrayloterismtinetgrybyteEsstihnegtub.yeEt ashl.e, twuh. eottaels.t,ewdhaosetersietesdofaSsIeBrielsecotfroSlIyBtelrecatcrtoilvyiteierseancd- −3 tfiovuitniedstahnadt1fomunodldthmat1NmaoClldOm4 inN1a:1CElOC4/iDnE1C:1uEnCd/eDrEgoCeusnexdoetrhgeoremsiecxroetahcetriomniscsrteaarctitniognast −3 ◦◦◦ s7ta0rtCing[2a4t].7A0b°oCve[2640]. ACb, tohveec6a0pa°cCit,ythfellcwapitahciitnycrfeallswinigthteimncprerastiunrge,taenmdpaetra8t0urCe, athnedcaetll had a similar capacity to that measured at 25 ◦C. Furthermore, with a rising temperature, 80 °C, the cell had a similar capacity to that measured at 25 °C. Furthermore, with a rising the first cycle Coulombic efficiency fell and the 80 ◦C cell had the lowest first cycle CE temperature, the first cycle Coulombic efficiency fell and the 80 °C cell had the lowest first in the series at around 66%. The low first cycle efficiency can be attributed to electrolyte cycle CE in the series at around 66%. The low first cycle efficiency can be attributed to degradation in the formation of the SEI. The loss of capacity in subsequent cycling has a electrolyte degradation in the formation of the SEI. The loss of capacity in subsequent similar slope at all temperatures above 25 ◦C, suggesting good thermal stability of the SEI cycling has a similar slope at all temperatures above 25 °C, suggesting good thermal sta- layers on the time scale of these experiments, although continuous electrolyte degradation bility of the SEI layers on the time scale of these experiments, although continuous elec- may compromise longer term performance. trolyte degradation may compromise longer term performance. To investigate the mechanism of charge storage using HC at different temperatures, To investigate the mechanism of charge storage using HC at different temperatures, the galvanostatic cycling profiles are plotted in Figure 5, which displays the relationship the galvanostatic cycling profiles are plotted in Figure 5, which displays the relationship between potential and capacity directly. At room temperature, there are two obvious regions in the potential plot during the first reduction that are related to the adsorption- intercalation mechanism of HC. The sloped region above 0.1 V is often referred to as the high potential plateau and contains around 100 mA h g−1 capacity due to sodium adsorption on the surfaces of HC nanopores [36–39]. The long, flat plateau region between 0.1 and 0.001 V corresponds to intercalation of sodium into HC layers and displays a 200 mA h g−1 ◦PDF Image | Temperature Dependence of Hard Carbon Sodium Half-Cells
PDF Search Title:
Temperature Dependence of Hard Carbon Sodium Half-CellsOriginal File Name Searched:
batteries_08_00108_v2.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |