
PDF Publication Title:
Text from PDF Page: 004
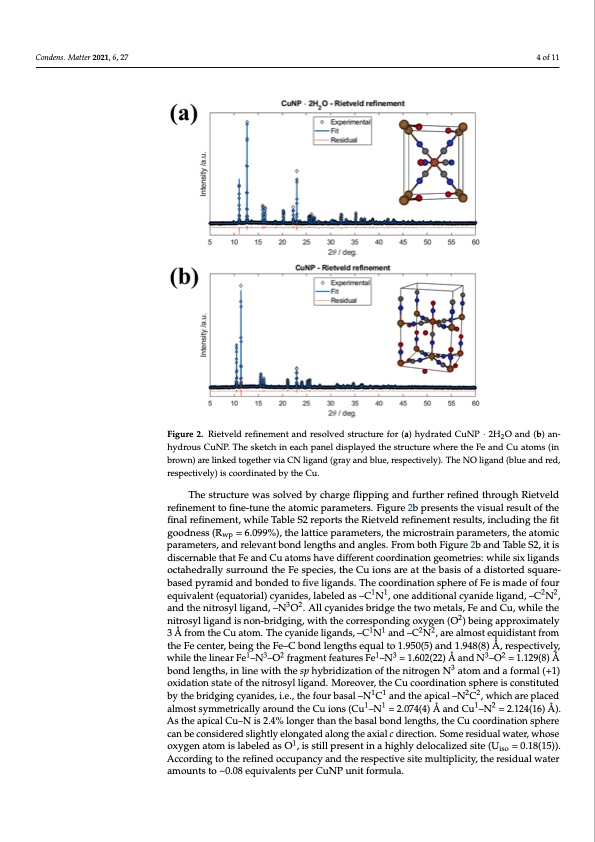
Condens. Matter 2021, 6, 27 4 of 11 Condens. Matter 2021, 6, x FOR PEER REVIEW 4 of 11 Fiigurree22..RRieitevtevledldrerfeinfienmemenetnatnadnrdesroeslvoelvdesdtrsutcrtucrteufroerf(oar)(hay)dhryadterdatCeduNCPuN∙ 2PH·2O2HanOd (abn)dan(bh)ya-n- 2 drous CuNP. The sketch in each panel displayed the structure where the Fe and Cu atoms (in hydrous CuNP. The sketch in each panel displayed the structure where the Fe and Cu atoms (in brown) are linked together via CN ligand (gray and blue, respectively). The NO ligand (blue and brown) are linked together via CN ligand (gray and blue, respectively). The NO ligand (blue and red, red, respectively) is coordinated by the Cu. respectively) is coordinated by the Cu. Analogous data analysis was carried out for the anhydrous CuNP structure. First, The structure was solved by charge flipping and further refined through Rietveld the cell was indexed, then Pawley refinement was carried out on the indexed cell, followed refinement to fine-tune the atomic parameters. Figure 2b presents the visual result of the bfiyncahl arergfieneflmipepnint,gwshtriulecTtuarbelesoSl2urtieopno,ratsndthRe iRetivetevldeldrerfienfienmemenetnwt aressfuinltasl,lyindcloundei.nTghtehecefillt wgoasodsuncecsess(sRfully=i6n.d0e9x9e%d),inthtehleatteictreapgaornaml Ie4tmerms, tshpeacmeigcrosutpra,iannpdatrhaemseotelvrse,dthsetrautcotmuriec wp mpartachmeedtewrse,lalnwditrheltehveanretpborntdedleonngeth[s15a]n.dFiagnugrlesS.3FreopmorbtostthFeibgeusrtef2itboafntdheTaPbalwe Sle2y, irtei-s fdinisecmerenatbcloentdhautcFtedabnyduCsuinagtoamgesnhearvaelidzeifdfeμresntrtacionomrdoidnealt,iownhgicehombettreiresd:ewschrilbeesixthleigpaenadks sohcatpaheeodfrdailflfyerseunrtroruefnledctihoensFe(csfp. SeIc)i.eTs,htehfeitCguooiodnsesasreasastotchieatbedaswisiothf athdeisPtaowrtledy srqefuianree-- mbaesnetdopnytrhaemaindhaynddrobuosnCduedNtPosfitrvuecltiugraenidsss.aTtihsefycionogr,dwinitahtioanRspwhpefarectofrFeeqiusamltaod5e.9o2f2f%ou.r 11 22 equiTvahlenstr(uecqtuarteorwials) csyoalvneidebs,ylacbhealregdeafsli–pCpinNg,aonnde faudrdthiteiornraelficnyeadnitdheroliuggahndR,i–eCtveNld, 32 raenfidnethmeenittrtosfyinleli-gtuande,t–hNeaOtom.Aicllpcayramniedtesrsb.rFidiguerteh2ebtwproemseentaslsth,FeevaisnudalCrue,suwlhtiolfethe 2 fninitarlorseyflinliegmanednti,swnohnil-ebTriadbglienSg2, wreipthorthtsetchoerrReiseptvoenldinregfionxeymgent(Ores)ubltesi,nigncalpupdrionxgimthaetefliyt 11 22 g3oÅodfnroemsst(hRewCpu=a6t.o0m99.%T)h,ethcyealnaitdtieceligpanrdams,e–tCersN,thaendm–iCcroNstr,aairnepaalmraomsteeteqrusi,dtihsteanatofmroimc the Fe center, being the Fe–C bond lengths equal to 1.950(5) and 1.948(8) Å, respectively, parameters, and relevant bond lengths and angles. From both Figure 2b and Table S2, it while the linear Fe1–N3–O2 fragment features Fe1–N3 = 1.602(22) Å and N3–O2 = 1.129(8) Å is discernable that Fe and Cu atoms have different coordination geometries: while six lig- bond lengths, in line with the sp hybridization of the nitrogen N3 atom and a formal (+1) ands octahedrally surround the Fe species, the Cu ions are at the basis of a distorted oxidation state of the nitrosyl ligand. Moreover, the Cu coordination sphere is constituted square-based pyramid and bonded to five ligands. The coordination sphere of Fe is made by the bridging cyanides, i.e., the four basal –N1C1 and1 th1e apical –N2C2, which are placed of four equivalent (equatorial) cyanides, labeled as –C N , one additional cyanide ligand, alm2o2stsymmetricallyaroundtheCu3 io2ns(Cu1–N1=2.074(4)ÅandCu1–N2=2.124(16)Å). –C N , and the nitrosyl ligand, –N O . All cyanides bridge the two metals, Fe and Cu, AstheapicalCu–Nis2.4%longerthanthebasalbondlengths,theCucoordina2tionsphere while the nitrosyl ligand is non-bridging, with the corresponding oxygen (O ) being ap- canbeconsideredslightlyelongatedalongtheaxialcdirection.S1om1eresidu2al2water,whose proximately 3 Å from the Cu atom. The cyanide ligands, –C N and –C N , are almost oxygen atom is labeled as O1, is still present in a highly delocalized site (U = 0.18(15)). equidistant from the Fe center, being the Fe–C bond lengths equal to 1.950(5) and 1.948(8) According to the refined occupancy and the respective site multiplicity, the residual water Å, respectively, while the linear Fe1–N3–O2 fragment features Fe1–N3 = 1.602(22) Å and N3– amounts to ~0.08 equivalents per CuNP unit formula. O2 = 1.129(8) Å bond lengths, in line with the sp hybridization of the nitrogen N3 atom and isoPDF Image | Cross-Investigation on Copper Nitroprusside: Combining XRD and XAS
PDF Search Title:
Cross-Investigation on Copper Nitroprusside: Combining XRD and XASOriginal File Name Searched:
condensedmatter-06-00027-v2.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |