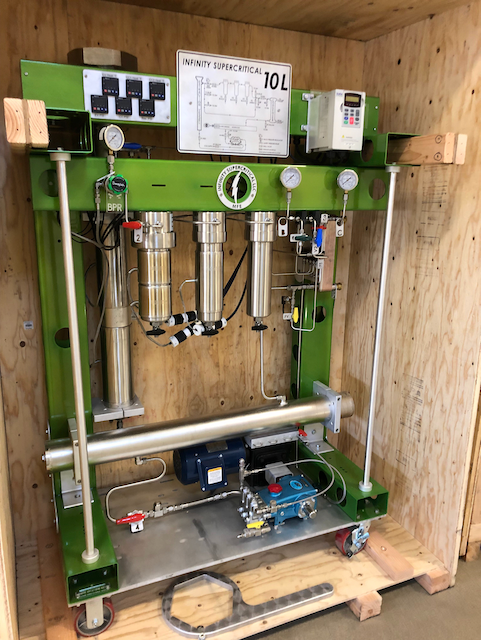
The Infinity Supercritical System May be Used for Vapor Deposition of Sulfur on Carbon Nanofiber to Experiment with production of Monoclinic Gamma-phase Sulfur for battery development
Lithium Sulfur Battery Research using Vapor Deposition of Sulfur on Carbon Nanofiber
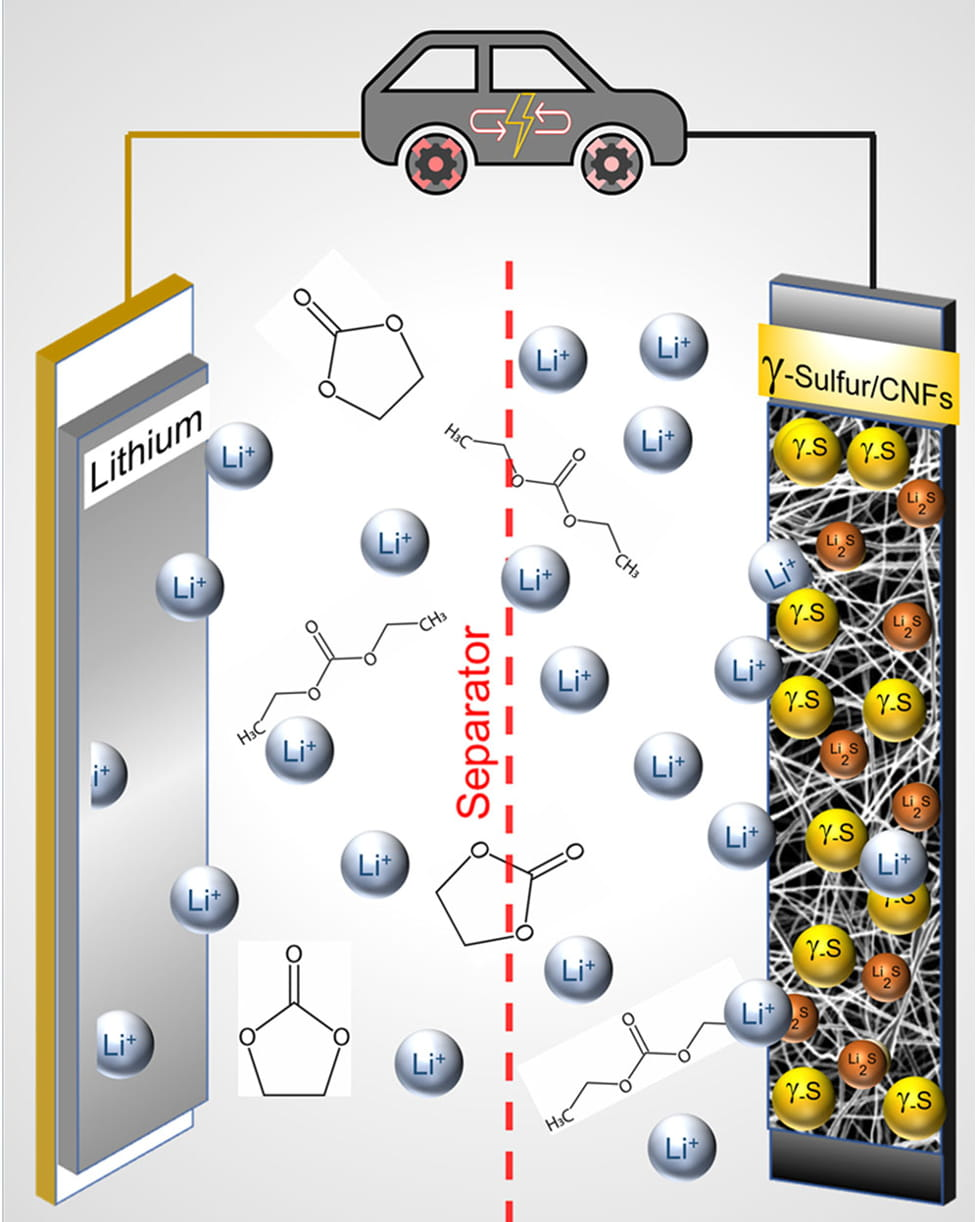
Lithium Sulfur Battery Research using Vapor Deposition of Sulfur on Carbon Nanofiber: One interesting application may be for the research and production of lithium sulfur batteries. Using CO2 may be one possible route to commercialization of production. ( The team found that during the process of depositing sulfur on the carbon nanofiber surface, changing it from a gas to a solid, it crystallized in an unexpected way, forming a slight variation of the element, called monoclinic gamma-phase sulfur - link below.)The modular construction of the system allow easy integration for new technology developments, and multi-role add-ons. The heart of the system is the phase change liquid pumping techniques, flow bar, and tribo effect electrostatic precipitation collection system. Many of these deployed technologies were developed by Infinity since 2015 making this system the most advanced in the industry. More than 100 of these commercial systems have been built and out around the world. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur, which was first prepared by F.W. Muthmann in 1890. Search PublicationsBreakthrough in Cathode Chemistry Clears Path for Lithium-Sulfur Batteries Commercial Viability
Supercritical Fluid Deposition Of Thin Metal Films: Kinetics, Mechanics And Applications