
PDF Publication Title:
Text from PDF Page: 002
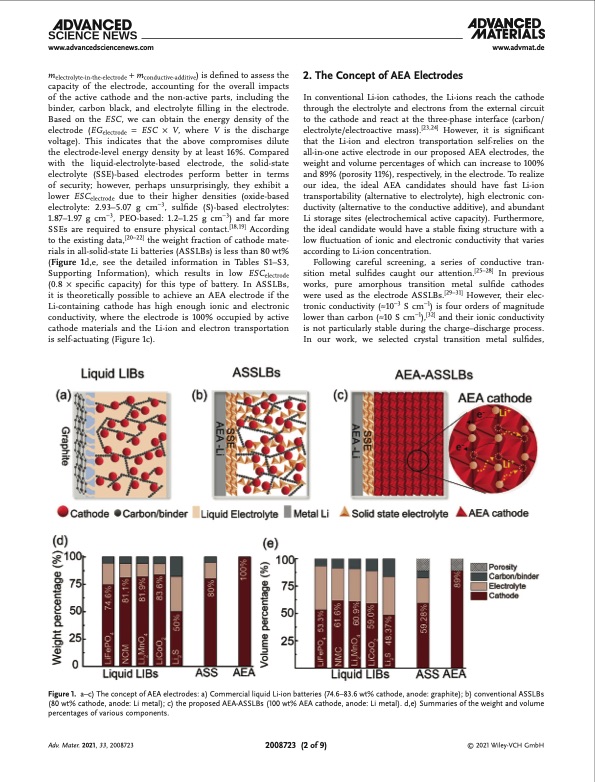
www.advancedsciencenews.com www.advmat.de melectrolyte-in-the-electrode + mconductive-additive) is defined to assess the capacity of the electrode, accounting for the overall impacts of the active cathode and the non-active parts, including the binder, carbon black, and electrolyte filling in the electrode. Based on the ESC, we can obtain the energy density of the electrode (EGelectrode = ESC × V, where V is the discharge voltage). This indicates that the above compromises dilute the electrode-level energy density by at least 16%. Compared with the liquid-electrolyte-based electrode, the solid-state electrolyte (SSE)-based electrodes perform better in terms of security; however, perhaps unsurprisingly, they exhibit a lower ESCelectrode due to their higher densities (oxide-based electrolyte: 2.93–5.07 g cm−3, sulfide (S)-based electrolytes: 1.87–1.97 g cm−3, PEO-based: 1.2–1.25 g cm−3) and far more SSEs are required to ensure physical contact.[18,19] According to the existing data,[20–22] the weight fraction of cathode mate- rials in all-solid-state Li batteries (ASSLBs) is less than 80 wt% (Figure 1d,e, see the detailed information in Tables S1–S3, Supporting Information), which results in low ESCelectrode (0.8 × specific capacity) for this type of battery. In ASSLBs, it is theoretically possible to achieve an AEA electrode if the Li-containing cathode has high enough ionic and electronic conductivity, where the electrode is 100% occupied by active cathode materials and the Li-ion and electron transportation is self-actuating (Figure 1c). 2. The Concept of AEA Electrodes In conventional Li-ion cathodes, the Li-ions reach the cathode through the electrolyte and electrons from the external circuit to the cathode and react at the three-phase interface (carbon/ electrolyte/electroactive mass).[23,24] However, it is significant that the Li-ion and electron transportation self-relies on the all-in-one active electrode in our proposed AEA electrodes, the weight and volume percentages of which can increase to 100% and 89% (porosity 11%), respectively, in the electrode. To realize our idea, the ideal AEA candidates should have fast Li-ion transportability (alternative to electrolyte), high electronic con- ductivity (alternative to the conductive additive), and abundant Li storage sites (electrochemical active capacity). Furthermore, the ideal candidate would have a stable fixing structure with a low fluctuation of ionic and electronic conductivity that varies according to Li-ion concentration. Following careful screening, a series of conductive tran- sition metal sulfides caught our attention.[25–28] In previous works, pure amorphous transition metal sulfide cathodes were used as the electrode ASSLBs.[29–31] However, their elec- tronic conductivity (≈10−3 S cm−1) is four orders of magnitude lower than carbon (≈10 S cm−1),[32] and their ionic conductivity is not particularly stable during the charge–discharge process. In our work, we selected crystal transition metal sulfides, Figure 1. a–c) The concept of AEA electrodes: a) Commercial liquid Li-ion batteries (74.6–83.6 wt% cathode, anode: graphite); b) conventional ASSLBs (80 wt% cathode, anode: Li metal); c) the proposed AEA-ASSLBs (100 wt% AEA cathode, anode: Li metal). d,e) Summaries of the weight and volume percentages of various components. Adv. Mater. 2021, 33, 2008723 2008723 (2 of 9) © 2021 Wiley-VCH GmbHPDF Image | Dense All-Electrochem-Active Electrodes for All-Solid-State Lithium Batteries
PDF Search Title:
Dense All-Electrochem-Active Electrodes for All-Solid-State Lithium BatteriesOriginal File Name Searched:
Li21LiuAM.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |