
PDF Publication Title:
Text from PDF Page: 004
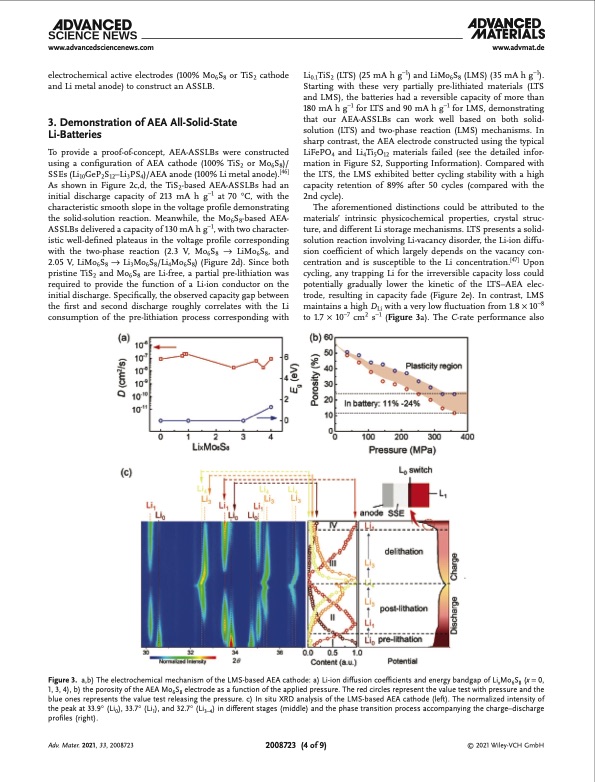
www.advancedsciencenews.com www.advmat.de electrochemical active electrodes (100% Mo6S8 or TiS2 cathode and Li metal anode) to construct an ASSLB. 3. Demonstration of AEA All-Solid-State Li-Batteries To provide a proof-of-concept, AEA-ASSLBs were constructed using a configuration of AEA cathode (100% TiS2 or Mo6S8)/ SSEs (Li10GeP2S12–Li3PS4)/AEA anode (100% Li metal anode).[46] As shown in Figure 2c,d, the TiS2-based AEA-ASSLBs had an initial discharge capacity of 213 mA h g−1 at 70 °C, with the characteristic smooth slope in the voltage profile demonstrating the solid-solution reaction. Meanwhile, the Mo6S8-based AEA- ASSLBs delivered a capacity of 130 mA h g−1, with two character- istic well-defined plateaus in the voltage profile corresponding with the two-phase reaction (2.3 V, Mo6S8 → LiMo6S8, and 2.05 V, LiMo6S8 → Li3Mo6S8/Li4Mo6S8) (Figure 2d). Since both pristine TiS2 and Mo6S8 are Li-free, a partial pre-lithiation was required to provide the function of a Li-ion conductor on the initial discharge. Specifically, the observed capacity gap between the first and second discharge roughly correlates with the Li consumption of the pre-lithiation process corresponding with Li0.1TiS2 (LTS) (25 mA h g−1) and LiMo6S8 (LMS) (35 mA h g−1). Starting with these very partially pre-lithiated materials (LTS and LMS), the batteries had a reversible capacity of more than 180 mA h g−1 for LTS and 90 mA h g−1 for LMS, demonstrating that our AEA-ASSLBs can work well based on both solid- solution (LTS) and two-phase reaction (LMS) mechanisms. In sharp contrast, the AEA electrode constructed using the typical LiFePO4 and Li4Ti5O12 materials failed (see the detailed infor- mation in Figure S2, Supporting Information). Compared with the LTS, the LMS exhibited better cycling stability with a high capacity retention of 89% after 50 cycles (compared with the 2nd cycle). The aforementioned distinctions could be attributed to the materials’ intrinsic physicochemical properties, crystal struc- ture, and different Li storage mechanisms. LTS presents a solid- solution reaction involving Li-vacancy disorder, the Li-ion diffu- sion coefficient of which largely depends on the vacancy con- centration and is susceptible to the Li concentration.[47] Upon cycling, any trapping Li for the irreversible capacity loss could potentially gradually lower the kinetic of the LTS–AEA elec- trode, resulting in capacity fade (Figure 2e). In contrast, LMS maintains a high DLi with a very low fluctuation from 1.8 × 10−8 to 1.7 × 10−7 cm2 s−1 (Figure 3a). The C-rate performance also Figure 3. a,b) The electrochemical mechanism of the LMS-based AEA cathode: a) Li-ion diffusion coefficients and energy bandgap of LixMo6S8 (x = 0, 1, 3, 4), b) the porosity of the AEA Mo6S8 electrode as a function of the applied pressure. The red circles represent the value test with pressure and the blue ones represents the value test releasing the pressure. c) In situ XRD analysis of the LMS-based AEA cathode (left). The normalized intensity of the peak at 33.9° (Li0), 33.7° (Li1), and 32.7° (Li3–4) in different stages (middle) and the phase transition process accompanying the charge–discharge profiles (right). Adv. Mater. 2021, 33, 2008723 2008723 (4 of 9) © 2021 Wiley-VCH GmbHPDF Image | Dense All-Electrochem-Active Electrodes for All-Solid-State Lithium Batteries
PDF Search Title:
Dense All-Electrochem-Active Electrodes for All-Solid-State Lithium BatteriesOriginal File Name Searched:
Li21LiuAM.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |