
PDF Publication Title:
Text from PDF Page: 002
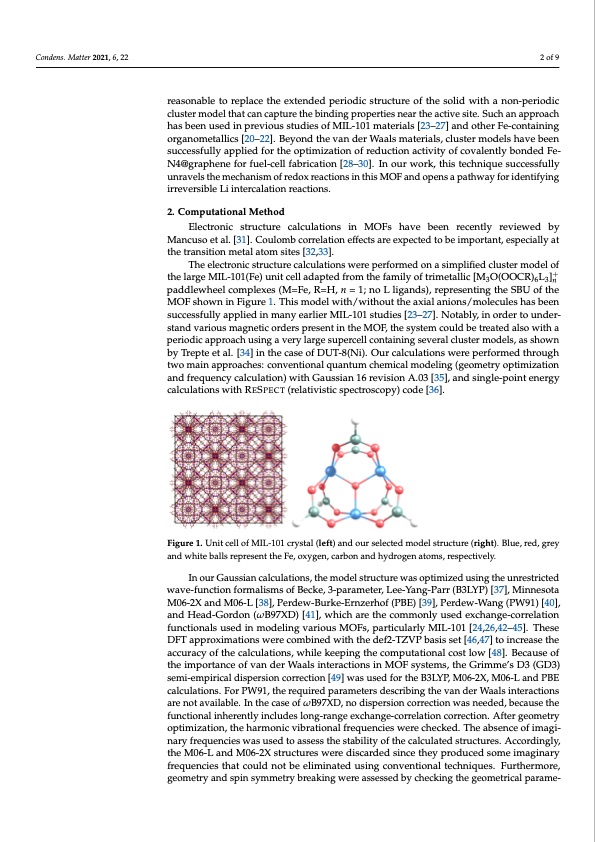
Condens. Matter 2021, 6, 22 2 of 9 reasonable to replace the extended periodic structure of the solid with a non-periodic cluster model that can capture the binding properties near the active site. Such an approach has been used in previous studies of MIL-101 materials [23–27] and other Fe-containing organometallics [20–22]. Beyond the van der Waals materials, cluster models have been successfully applied for the optimization of reduction activity of covalently bonded Fe- N4@graphene for fuel-cell fabrication [28–30]. In our work, this technique successfully unravels the mechanism of redox reactions in this MOF and opens a pathway for identifying irreversible Li intercalation reactions. 2. Computational Method Electronic structure calculations in MOFs have been recently reviewed by Mancuso et al. [31]. Coulomb correlation effects are expected to be important, especially at the transition metal atom sites [32,33]. The electronic structure calculations were performed on a simplified cluster model of the large MIL-101(Fe) unit cell adapted from the family of trimetallic [M3O(OOCR)6L3]+n paddlewheel complexes (M=Fe, R=H, n = 1; no L ligands), representing the SBU of the MOF shown in Figure 1. This model with/without the axial anions/molecules has been successfully applied in many earlier MIL-101 studies [23–27]. Notably, in order to under- stand various magnetic orders present in the MOF, the system could be treated also with a periodic approach using a very large supercell containing several cluster models, as shown by Trepte et al. [34] in the case of DUT-8(Ni). Our calculations were performed through two main approaches: conventional quantum chemical modeling (geometry optimization and frequency calculation) with Gaussian 16 revision A.03 [35], and single-point energy calculations with RESPECT (relativistic spectroscopy) code [36]. Figure 1. The unit cFeilglusrteru1c.tuUrneitocfeMllIoLf-M10IL1-(1l0e1ftc),ryasntdalth(lefste)laenctdedoumrosdeleelc(treidghmt)o.dTehlestbrluucet,ured(,rigrhety). Blue, red, grey and white balls respaencdtivwehlyiterebparlelserenpt rtehseenFte,thoexFyeg,eonx, ycgaerbno, cnaarbnodnhaynddrohgyednroatgoemn sa.toms, respectively. In the conventional calculations, the model structure was optimized using the unrestricted wave In our Gaussian calculations, the model structure was optimized using the unrestricted function formalisms of the B3LYP,1 M06-2X,2 M06-L,3 PBEPBE,4 PW91PW91,5 and ωB97XD6 wave-function formalisms of Becke, 3-parameter, Lee-Yang-Parr (B3LYP) [37], Minnesota methods, which are the methods commonly used in modeling of various MOFs, particularly MIL- M06-2X and M06-L [38], Perdew-Burke-Ernzerhof (PBE) [39], Perdew-Wang (PW91) [40], 101.78910 11 1213 14151617181920 21222324 252627(each separated set of references refers to one of the methods; and Head-Gordon (ωB97XD) [41], which are the commonly used exchange-correlation PBEPBE and PBE0; just the first wB97xD ref is for MIL-101). These methods were combined with functionals used in modeling various MOFs, particularly MIL-101 [24,26,42–45]. These the def2-TZVP2829 basis set to increase the accuracy of the calculations while keeping the DFT approximations were combined with the def2-TZVP basis set [46,47] to increase the computational cost low.30 Because of the importance of van der Waals interactions in MOF systems, accuracy of the calculations, while keeping the computational cost low [48]. Because of the Grimme’s D3 (GD3) semi-empirical dispersion correction31 was used for the B3LYP, M06-2X, the importance of van der Waals interactions in MOF systems, the Grimme’s D3 (GD3) M06-L and PBEPBE calculations. For PW91PW91, the required parameters were missing. In the semi-empirical dispersion correction [49] was used for the B3LYP, M06-2X, M06-L and PBE case of ωB97xD, no dispersion correction was needed because the functional inherently includes calculations. For PW91, the required parameters describing the van der Waals interactions long-range exchange-correlation correction.323334 After geometry optimization, the harmonic are not available. In the case of ωB97XD, no dispersion correction was needed, because the vibrational frequencies were checked, and the lack of any imaginary frequency approved the stability functional inherently includes long-range exchange-correlation correction. After geometry of the obtained structures. Accordingly, the M06-L and M06-2X calculations were retarded after optimization, the harmonic vibrational frequencies were checked. The absence of imagi- observing some imaginary frequencies that could not be eliminated using conventional techniques. nary frequencies was used to assess the stability of the calculated structures. Accordingly, Furthermore, geometry and spin symmetry breaking were respectively assessed by checking the the M06-L and M06-2X structures were discarded since they produced some imaginary geometrical parameters and the total spin values before (S2) and after (S2A) annihilation of the highest frequencies that could not be eliminated using conventional techniques. Furthermore, spin contaminant. Finally, to calculate the electrochemical potential as a Li-battery cathode, the geometry and spin symmetry breaking were assessed by checking the geometrical parame- interaction between one to three Li+ ions with the model clusters (which results in the reduction of the Fe3+ metal centers to Fe2+)35 was simulated by inserting the Li+ cations in adjacency of the metal centers and optimizing the resultant structures. For the ReSpect calculations, which provide high accuracy and efficiently for studying materials with heavier atoms (such as transition metals) based on the four-component fully relativistic DiracPDF Image | Electrochemical Potential MIL-101(Fe) as Cathode Material in Li-Ion Batteries
PDF Search Title:
Electrochemical Potential MIL-101(Fe) as Cathode Material in Li-Ion BatteriesOriginal File Name Searched:
condensedmatter-06-00022.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |