
PDF Publication Title:
Text from PDF Page: 004
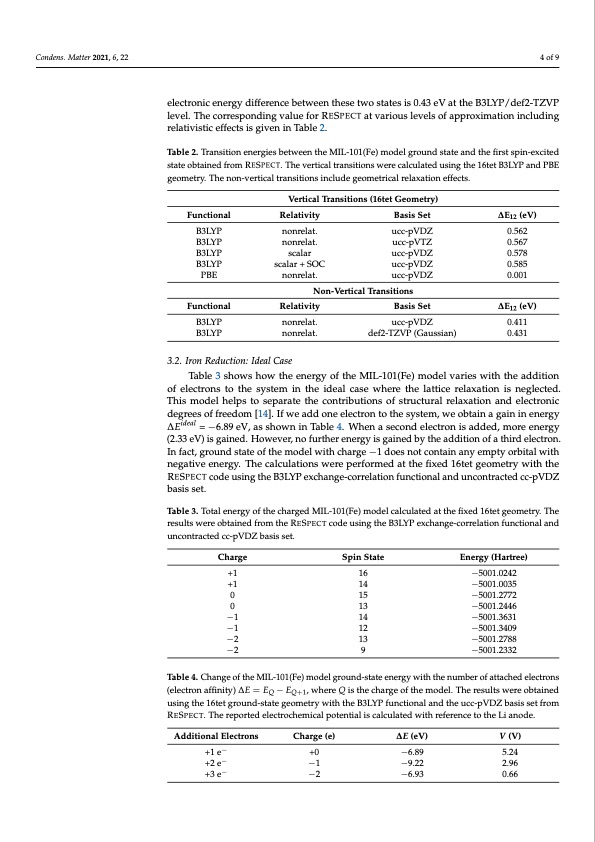
Condens. Matter 2021, 6, 22 4 of 9 electronic energy difference between these two states is 0.43 eV at the B3LYP/def2-TZVP level. The corresponding value for RESPECT at various levels of approximation including relativistic effects is given in Table 2. Table 2. Transition energies between the MIL-101(Fe) model ground state and the first spin-excited state obtained from RESPECT. The vertical transitions were calculated using the 16tet B3LYP and PBE geometry. The non-vertical transitions include geometrical relaxation effects. Functional B3LYP B3LYP B3LYP B3LYP PBE Functional B3LYP B3LYP Relativity nonrelat. nonrelat. scalar scalar + SOC nonrelat. Basis Set ucc-pVDZ ucc-pVTZ ucc-pVDZ ucc-pVDZ ucc-pVDZ ∆E12 (eV) 0.562 0.567 0.578 0.585 0.001 ∆E12 (eV) 0.411 0.431 Vertical Transitions (16tet Geometry) Relativity nonrelat. nonrelat. Basis Set ucc-pVDZ def2-TZVP (Gaussian) Non-Vertical Transitions 3.2. Iron Reduction: Ideal Case Table 3 shows how the energy of the MIL-101(Fe) model varies with the addition of electrons to the system in the ideal case where the lattice relaxation is neglected. This model helps to separate the contributions of structural relaxation and electronic degrees of freedom [14]. If we add one electron to the system, we obtain a gain in energy ∆Eideal = −6.89 eV, as shown in Table 4. When a second electron is added, more energy (2.33 eV) is gained. However, no further energy is gained by the addition of a third electron. In fact, ground state of the model with charge −1 does not contain any empty orbital with negative energy. The calculations were performed at the fixed 16tet geometry with the RESPECT code using the B3LYP exchange-correlation functional and uncontracted cc-pVDZ basis set. Table 3. Total energy of the charged MIL-101(Fe) model calculated at the fixed 16tet geometry. The results were obtained from the RESPECT code using the B3LYP exchange-correlation functional and uncontracted cc-pVDZ basis set. Charge Spin State Energy (Hartree) −5001.0242 −5001.0035 −5001.2772 −5001.2446 −5001.3631 −5001.3409 −5001.2788 −5001.2332 +1 16 +1 14 0 15 0 13 −1 14 −1 12 −2 13 −2 9 Table 4. Change of the MIL-101(Fe) model ground-state energy with the number of attached electrons (electron affinity) ∆E = EQ − EQ+1, where Q is the charge of the model. The results were obtained using the 16tet ground-state geometry with the B3LYP functional and the ucc-pVDZ basis set from RESPECT. The reported electrochemical potential is calculated with reference to the Li anode. Additional Electrons +1 e− +2 e− +3 e− Charge (e) +0 −1 −2 ∆E (eV) −6.89 −9.22 −6.93 V (V) 5.24 2.96 0.66PDF Image | Electrochemical Potential MIL-101(Fe) as Cathode Material in Li-Ion Batteries
PDF Search Title:
Electrochemical Potential MIL-101(Fe) as Cathode Material in Li-Ion BatteriesOriginal File Name Searched:
condensedmatter-06-00022.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |