
PDF Publication Title:
Text from PDF Page: 083
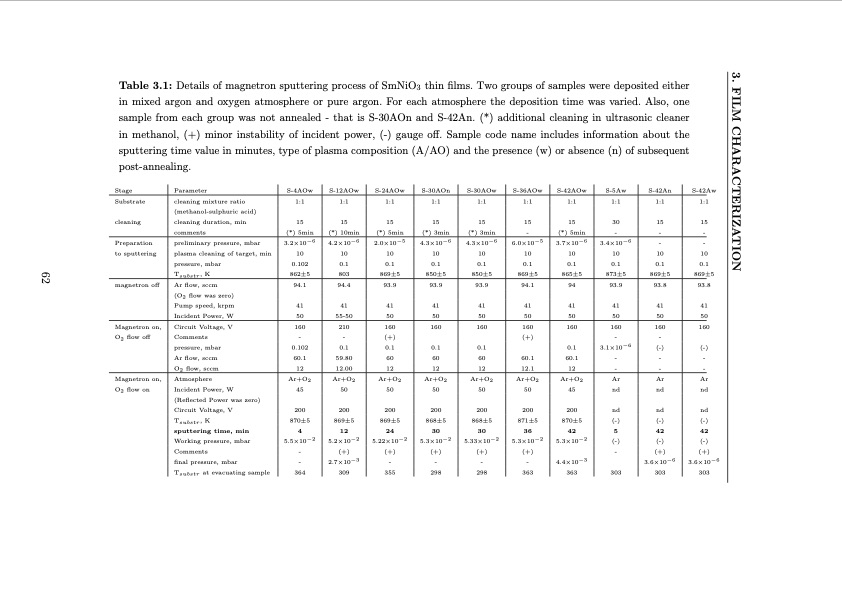
3. FILM CHARACTERIZATION 62 Table 3.1: Details of magnetron sputtering process of SmNiO3 thin films. Two groups of samples were deposited either in mixed argon and oxygen atmosphere or pure argon. For each atmosphere the deposition time was varied. Also, one sample from each group was not annealed - that is S-30AOn and S-42An. (*) additional cleaning in ultrasonic cleaner in methanol, (+) minor instability of incident power, (-) gauge off. Sample code name includes information about the sputtering time value in minutes, type of plasma composition (A/AO) and the presence (w) or absence (n) of subsequent post-annealing. Stage Substrate Parameter cleaning mixture ratio (methanol-sulphuric acid) cleaning duration, min comments preliminary pressure, mbar plasma cleaning of target, min pressure, mbar Tsubstr , K Ar flow, sccm (O2 flow was zero) Pump speed, krpm Incident Power, W Circuit Voltage, V Comments pressure, mbar Ar flow, sccm O2 flow, sccm Atmosphere Incident Power, W (Reflected Power was zero) Circuit Voltage, V Tsubstr, K sputtering time, min Working pressure, mbar Comments final pressure, mbar Tsubstr at evacuating sample S-4AOw S-12AOw S-24AOw S-30AOn S-30AOw 1:1 1:1 S-36AOw 1:1 S-42AOw S-5Aw 1:1 1:1 S-42An S-42Aw 1:1 1:1 cleaning 15 (*) 5min 3.2×10−6 10 0.102 862±5 94.1 15 15 (*) 10min (*) 5min 4.2×10−6 2.0×10−5 10 10 0.1 0.1 15 15 (*) 3min (*) 3min 4.3×10−6 4.3×10−6 10 10 0.1 0.1 850±5 850±5 93.9 93.9 15 - 6.0×10−5 10 0.1 869±5 94.1 15 30 (*) 5min - 15 15 - - - - 10 10 0.1 0.1 869±5 869±5 93.8 93.8 Preparation to sputtering 3.7×10−6 3.4×10−6 10 10 0.1 0.1 865±5 873±5 magnetron off 803 869±5 94.4 93.9 94 93.9 Magnetron on, O2 flow off 41 50 160 - 0.102 60.1 12 Ar+O2 45 41 41 55-50 50 210 160 41 41 50 50 160 160 41 50 160 (+) 41 41 50 50 160 160 41 41 50 50 160 160 Magnetron on, O2 flow on 12.00 Ar+O2 Ar+O2 Ar+O2 Ar+O2 Ar+O2 Ar+O2 Ar Ar Ar nd nd nd 1:1 1:1 1:1 200 870±5 4 5.5×10−2 - - 364 50 200 50 50 50 50 45 200 200 200 200 200 nd nd nd (-) (-) (-) 5 42 42 (-) (-) (-) - (+) 0.1 0.1 - - 59.80 869±5 12 5.2×10−2 (+) 2.7×10−3 309 869±5 868±5 868±5 871±5 870±5 24 30 30 36 42 0.1 3.1×10−6 60 60 60 60.1 60.1 - 12 12 12 12.1 12 - (-) (-) - - - - 5.22×10−2 (+) - 355 5.3×10−2 5.33×10−2 5.3×10−2 (+) (+) (+) - - - 298 298 363 5.3×10−2 4.4×10−3 - (+) (+) 0.1 0.1 363 3.6×10−6 303 303 303 3.6×10−6PDF Image | Investigation of metal-insulator transition in magnetron sputtered samarium nickelate thin films
PDF Search Title:
Investigation of metal-insulator transition in magnetron sputtered samarium nickelate thin filmsOriginal File Name Searched:
Bilewska_Investigation_of_metal_insulator_transition_in_magnetron_sputtered_samarium.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |