
PDF Publication Title:
Text from PDF Page: 045
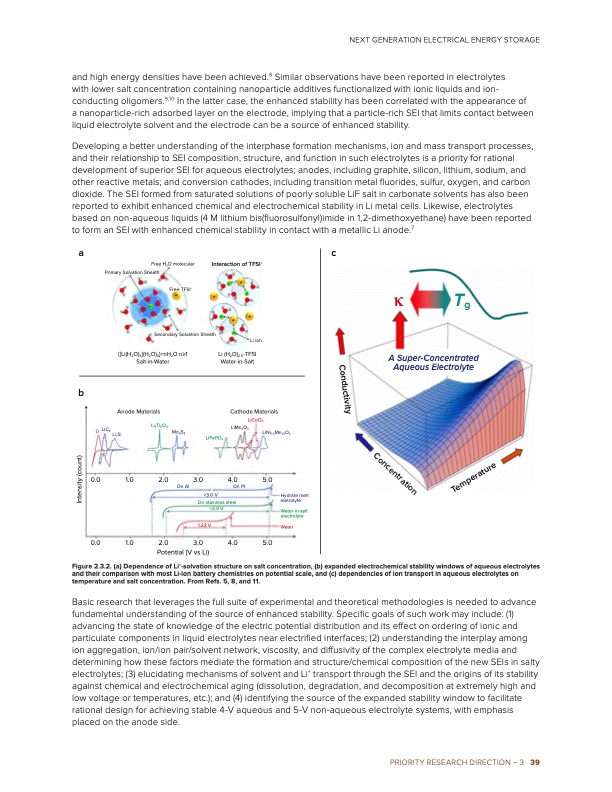
and high energy densities have been achieved.8 Similar observations have been reported in electrolytes with lower salt concentration containing nanoparticle additives functionalized with ionic liquids and ion- conducting oligomers.9,10 In the latter case, the enhanced stability has been correlated with the appearance of a nanoparticle-rich adsorbed layer on the electrode, implying that a particle-rich SEI that limits contact between liquid electrolyte solvent and the electrode can be a source of enhanced stability. Developing a better understanding of the interphase formation mechanisms, ion and mass transport processes, and their relationship to SEI composition, structure, and function in such electrolytes is a priority for rational development of superior SEI for aqueous electrolytes; anodes, including graphite, silicon, lithium, sodium, and other reactive metals; and conversion cathodes, including transition metal fluorides, sulfur, oxygen, and carbon dioxide. The SEI formed from saturated solutions of poorly soluble LiF salt in carbonate solvents has also been reported to exhibit enhanced chemical and electrochemical stability in Li metal cells. Likewise, electrolytes based on non-aqueous liquids (4 M lithium bis(fluorosulfonyl)imide in 1,2-dimethoxyethane) have been reported to form an SEI with enhanced chemical stability in contact with a metallic Li anode.7 ac Free H2O molecular Interaction of TFSI– NEXT GENERATION ELECTRICAL ENERGY STORAGE b Secondary Solvation Sheath {[Li(H2O)4](H2O)4}+nH2O n≥1 Salt-in-Water Anode Materials Li ion Li (H2O)2.5-TFSI Water-in-Salt Cathode Materials LiCoO2 Primary Solvation Sheath Free TFSI– κ Tg A Super-Concentrated Aqueous Electrolyte Li LiC6 LixSi 0.0 1.0 0.0 1.0 Li4Ti5O12 2.0 Mo6S8 On Al LiMn2O4 LiNi0.5Mn1.5O4 2.0 Potential (V vs Li) 3.0 LiFePO4 >3.0 V 4.0 5.0 On Pt On stainless steel ~3.0 V 1.23 V Hydrate melt eletrolyte Water-in-salt electrolyte Water 3.0 4.0 5.0 Figure 2.3.2. (a) Dependence of Li+-solvation structure on salt concentration, (b) expanded electrochemical stability windows of aqueous electrolytes and their comparison with most Li-ion battery chemistries on potential scale, and (c) dependencies of ion transport in aqueous electrolytes on temperature and salt concentration. From Refs. 5, 8, and 11. Basic research that leverages the full suite of experimental and theoretical methodologies is needed to advance fundamental understanding of the source of enhanced stability. Specific goals of such work may include: (1) advancing the state of knowledge of the electric potential distribution and its effect on ordering of ionic and particulate components in liquid electrolytes near electrified interfaces; (2) understanding the interplay among ion aggregation, ion/ion pair/solvent network, viscosity, and diffusivity of the complex electrolyte media and determining how these factors mediate the formation and structure/chemical composition of the new SEIs in salty electrolytes; (3) elucidating mechanisms of solvent and Li+ transport through the SEI and the origins of its stability against chemical and electrochemical aging (dissolution, degradation, and decomposition at extremely high and low voltage or temperatures, etc.); and (4) identifying the source of the expanded stability window to facilitate rational design for achieving stable 4-V aqueous and 5-V non-aqueous electrolyte systems, with emphasis placed on the anode side. PRIORITY RESEARCH DIRECTION – 3 39 Conductivity Intensity (count) Concentration TemperaturePDF Image | Next Generation Electrical Energy Storage
PDF Search Title:
Next Generation Electrical Energy StorageOriginal File Name Searched:
BRN-NGEES_rpt-low-res.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |