
PDF Publication Title:
Text from PDF Page: 048
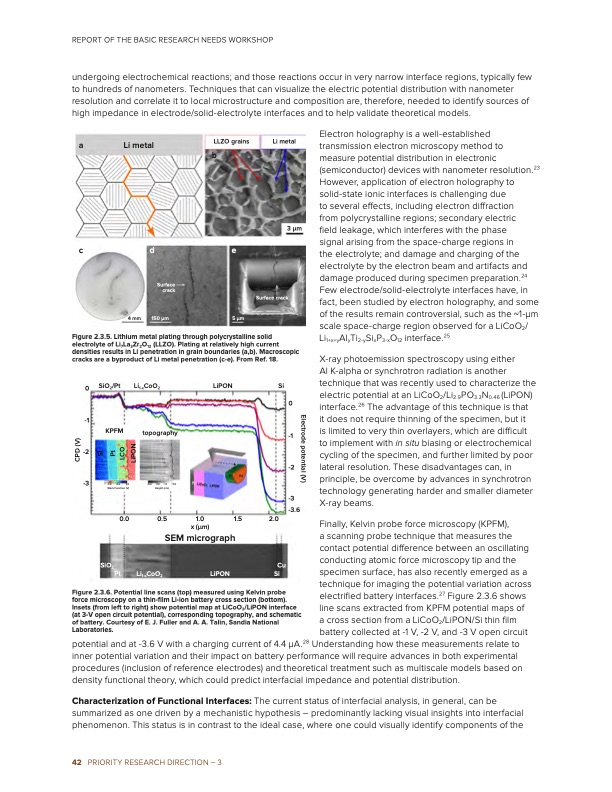
REPORT OF THE BASIC RESEARCH NEEDS WORKSHOP undergoing electrochemical reactions; and those reactions occur in very narrow interface regions, typically few to hundreds of nanometers. Techniques that can visualize the electric potential distribution with nanometer resolution and correlate it to local microstructure and composition are, therefore, needed to identify sources of high impedance in electrode/solid-electrolyte interfaces and to help validate theoretical models. a Li metal Li metal 3 μm Surface crack cde Surface 4 mm crack 150 μm 5 μm Figure 2.3.5. Lithium metal plating through polycrystalline solid electrolyte of Li7La3Zr2O12 (LLZO). Plating at relatively high current densities results in Li penetration in grain boundaries (a,b). Macroscopic cracks are a byproduct of Li metal penetration (c-e). From Ref. 18. LLZO grains b Electron holography is a well-established transmission electron microscopy method to measure potential distribution in electronic (semiconductor) devices with nanometer resolution.23 However, application of electron holography to solid-state ionic interfaces is challenging due to several effects, including electron diffraction from polycrystalline regions; secondary electric field leakage, which interferes with the phase signal arising from the space-charge regions in the electrolyte; and damage and charging of the electrolyte by the electron beam and artifacts and damage produced during specimen preparation.24 Few electrode/solid-electrolyte interfaces have, in fact, been studied by electron holography, and some of the results remain controversial, such as the ~1-μm scale space-charge region observed for a LiCoO2/ Li1+x+yAlyTi2-ySixP3-xO12 interface.25 X-ray photoemission spectroscopy using either Al K-alpha or synchrotron radiation is another technique that was recently used to characterize the electric potential at an LiCoO2/Li2.9PO3.3N0.46 (LiPON) interface.26 The advantage of this technique is that it does not require thinning of the specimen, but it is limited to very thin overlayers, which are difficult to implement with in situ biasing or electrochemical cycling of the specimen, and further limited by poor lateral resolution. These disadvantages can, in principle, be overcome by advances in synchrotron technology generating harder and smaller diameter -3 -3.6 0 SiO2/Pt -1 -2 -3 Li1-xCoO2 topography LiPON Si KPFM 0 -1 -2 -1.0 0.0 Work Function (V) 0.0 SiO2 Pt -20 -10 0 Height (nm) 1.0 1.0 X-ray beams. Finally, Kelvin probe force microscopy (KPFM), a scanning probe technique that measures the contact potential difference between an oscillating conducting atomic force microscopy tip and the specimen surface, has also recently emerged as a technique for imaging the potential variation across electrified battery interfaces.27 Figure 2.3.6 shows line scans extracted from KPFM potential maps of a cross section from a LiCoO /LiPON/Si thin film potential and at -3.6 V with a charging current of 4.4 μA.28 Understanding how these measurements relate to inner potential variation and their impact on battery performance will require advances in both experimental procedures (inclusion of reference electrodes) and theoretical treatment such as multiscale models based on density functional theory, which could predict interfacial impedance and potential distribution. Characterization of Functional Interfaces: The current status of interfacial analysis, in general, can be summarized as one driven by a mechanistic hypothesis – predominantly lacking visual insights into interfacial phenomenon. This status is in contrast to the ideal case, where one could visually identify components of the Li1-xCoO2 LiPON 0.5 SEM micrograph 2.0 Cu Si Figure 2.3.6. Potential line scans (top) measured using Kelvin probe force microscopy on a thin-film Li-ion battery cross section (bottom). Insets (from left to right) show potential map at LiCoO2/LiPON interface (at 3-V open circuit potential), corresponding topography, and schematic of battery. Courtesy of E. J. Fuller and A. A. Talin, Sandia National Laboratories. 2 battery collected at -1 V, -2 V, and -3 V open circuit 42 PRIORITY RESEARCH DIRECTION – 3 1.0 x (μm) 1.5 Electrode potential (V) CPD (V) SI Pt LCO LiPONPDF Image | Next Generation Electrical Energy Storage
PDF Search Title:
Next Generation Electrical Energy StorageOriginal File Name Searched:
BRN-NGEES_rpt-low-res.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |