
PDF Publication Title:
Text from PDF Page: 117
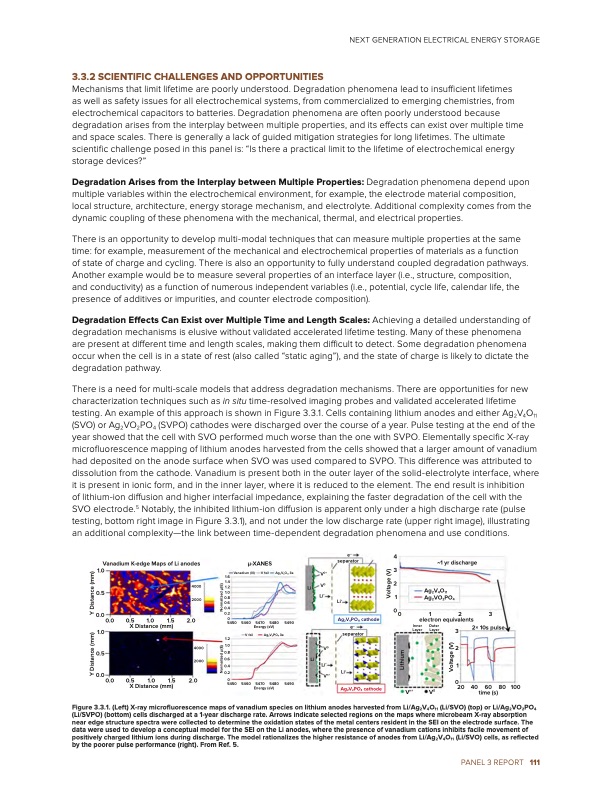
3.3.2 SCIENTIFIC CHALLENGES AND OPPORTUNITIES Mechanisms that limit lifetime are poorly understood. Degradation phenomena lead to insufficient lifetimes as well as safety issues for all electrochemical systems, from commercialized to emerging chemistries, from electrochemical capacitors to batteries. Degradation phenomena are often poorly understood because degradation arises from the interplay between multiple properties, and its effects can exist over multiple time and space scales. There is generally a lack of guided mitigation strategies for long lifetimes. The ultimate scientific challenge posed in this panel is: “Is there a practical limit to the lifetime of electrochemical energy storage devices?” Degradation Arises from the Interplay between Multiple Properties: Degradation phenomena depend upon multiple variables within the electrochemical environment, for example, the electrode material composition, local structure, architecture, energy storage mechanism, and electrolyte. Additional complexity comes from the dynamic coupling of these phenomena with the mechanical, thermal, and electrical properties. There is an opportunity to develop multi-modal techniques that can measure multiple properties at the same time: for example, measurement of the mechanical and electrochemical properties of materials as a function of state of charge and cycling. There is also an opportunity to fully understand coupled degradation pathways. Another example would be to measure several properties of an interface layer (i.e., structure, composition, and conductivity) as a function of numerous independent variables (i.e., potential, cycle life, calendar life, the presence of additives or impurities, and counter electrode composition). Degradation Effects Can Exist over Multiple Time and Length Scales: Achieving a detailed understanding of degradation mechanisms is elusive without validated accelerated lifetime testing. Many of these phenomena are present at different time and length scales, making them difficult to detect. Some degradation phenomena occur when the cell is in a state of rest (also called “static aging”), and the state of charge is likely to dictate the degradation pathway. There is a need for multi-scale models that address degradation mechanisms. There are opportunities for new characterization techniques such as in situ time-resolved imaging probes and validated accelerated lifetime testing. An example of this approach is shown in Figure 3.3.1. Cells containing lithium anodes and either Ag2V4O11 (SVO) or Ag2VO2PO4 (SVPO) cathodes were discharged over the course of a year. Pulse testing at the end of the year showed that the cell with SVO performed much worse than the one with SVPO. Elementally specific X-ray microfluorescence mapping of lithium anodes harvested from the cells showed that a larger amount of vanadium had deposited on the anode surface when SVO was used compared to SVPO. This difference was attributed to dissolution from the cathode. Vanadium is present both in the outer layer of the solid-electrolyte interface, where it is present in ionic form, and in the inner layer, where it is reduced to the element. The end result is inhibition of lithium-ion diffusion and higher interfacial impedance, explaining the faster degradation of the cell with the SVO electrode.5 Notably, the inhibited lithium-ion diffusion is apparent only under a high discharge rate (pulse testing, bottom right image in Figure 3.3.1), and not under the low discharge rate (upper right image), illustrating an additional complexity—the link between time-dependent degradation phenomena and use conditions. Vanadium K-edge Maps of Li anodes 1.0 μ-XANES e– 4 separator ~1 yr discharge Ag2V4O11 Ag2VO2PO4 0123 electron equivalents 0.5 0.0 1.0 0.5 0.0 1.4 4000 1.2 1.0 2000 0.8 0.6 0.4 0.2 2.0 50450 1.2 4000 1.0 0.8 Li 0.0 0.0 0.5 1.0 1.5 X Distance (mm) 5460 V foil 5470 Energy (eV) Li+ Ag2V4PO11 cathode e– separator 0.5 X Distance (mm) 1.0 1.5 2.0 50450 5460 5470 5480 5490 Energy (eV) 20 40 60 80 100 time (s) 2000 0.6 0.4 0.2 Li+ Vn+ 2 1 0 1.6 Vanadium (III) V foil Ag2V4O11 3e 5490 V0+ V0 Li+ V0 Li Li+ Ag V PO cathode 2 2 4 3 2 1 0 5480 Ag2V2PO4 3e Inner Layer Vn+ Outer Layer 3 2× 10s pulse Figure 3.3.1. (Left) X-ray microfluorescence maps of vanadium species on lithium anodes harvested from Li/Ag2V4O11 (Li/SVO) (top) or Li/Ag2VO2PO4 (Li/SVPO) (bottom) cells discharged at a 1-year discharge rate. Arrows indicate selected regions on the maps where microbeam X-ray absorption near edge structure spectra were collected to determine the oxidation states of the metal centers resident in the SEI on the electrode surface. The data were used to develop a conceptual model for the SEI on the Li anodes, where the presence of vanadium cations inhibits facile movement of positively charged lithium ions during discharge. The model rationalizes the higher resistance of anodes from Li/Ag2V4O11 (Li/SVO) cells, as reflected by the poorer pulse performance (right). From Ref. 5. NEXT GENERATION ELECTRICAL ENERGY STORAGE V0 PANEL 3 REPORT 111 Y Distance (mm) Y Distance (mm) Normalized μ(E) Normalized μ(E) Lithium Voltage (V) Voltage (V)PDF Image | Next Generation Electrical Energy Storage
PDF Search Title:
Next Generation Electrical Energy StorageOriginal File Name Searched:
BRN-NGEES_rpt-low-res.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |