
PDF Publication Title:
Text from PDF Page: 003
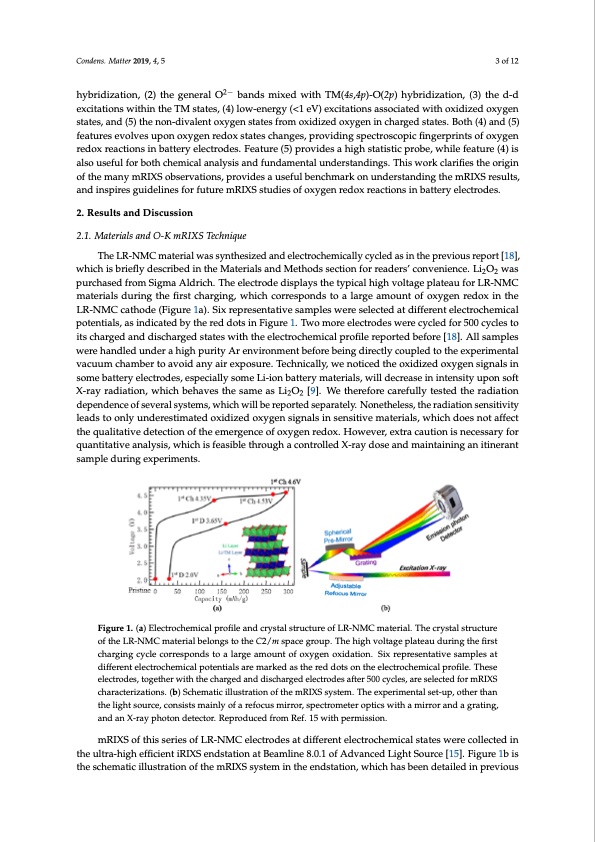
Condens. Matter 2019, 4, 5 3 of 12 hybridization, (2) the general O2− bands mixed with TM(4s,4p)-O(2p) hybridization, (3) the d-d excitations within the TM states, (4) low-energy (<1 eV) excitations associated with oxidized oxygen states, and (5) the non-divalent oxygen states from oxidized oxygen in charged states. Both (4) and (5) features evolves upon oxygen redox states changes, providing spectroscopic fingerprints of oxygen Condens. Matter 2019, 4, x FOR PEER REVIEW 3 of 12 redox reactions in battery electrodes. Feature (5) provides a high statistic probe, while feature (4) is hybridization, (3) the d-d excitations within the TM states, (4) low-energy (<1 eV) excitations also useful for both chemical analysis and fundamental understandings. This work clarifies the origin associated with oxidized oxygen states, and (5) the non-divalent oxygen states from oxidized oxygen of the many mRIXS observations, provides a useful benchmark on understanding the mRIXS results, in charged states. Both (4) and (5) features evolves upon oxygen redox states changes, providing and inspires guidelines for future mRIXS studies of oxygen redox reactions in battery electrodes. spectroscopic fingerprints of oxygen redox reactions in battery electrodes. Feature (5) provides a high statistic probe, while feature (4) is also useful for both chemical analysis and fundamental 2. Results and Discussion understandings. This work clarifies the origin of the many mRIXS observations, provides a useful benchmark on understanding the mRIXS results, and inspires guidelines for future mRIXS studies of 2.1. Materials and O-K mRIXS Technique oxygen redox reactions in battery electrodes. The LR-NMC material was synthesized and electrochemically cycled as in the previous report [18], 2. Results and Discussion which is briefly described in the Materials and Methods section for readers’ convenience. Li2O2 was purchased2.1f.rMomateSriiaglsmanadAOl-KdrmicRhIX. STThechenlieqcuterode displays the typical high voltage plateau for LR-NMC materials during the first charging, which corresponds to a large amount of oxygen redox in the The LR-NMC material was synthesized and electrochemically cycled as in the previous report LR-NMC[1c8a]t,hwohdiceh(iFsibgruierfley1dae)s.crSibiexdrienptrhesMenateartiiavlseasnadmMpeltehsodwsesercetisoenlefocrteredadaetrsd’icfofenrvenitenecle.cLtri2oOc2hemical was purchased from Sigma Aldrich. The electrode displays the typical high voltage plateau for potentials, as indicated by the red dots in Figure 1. Two more electrodes were cycled for 500 cycles to LR-NMC materials during the first charging, which corresponds to a large amount of oxygen redox in its charged and discharged states with the electrochemical profile reported before [18]. All samples the LR-NMC cathode (Figure 1a). Six representative samples were selected at different electrochemical were handled under a high purity Ar environment before being directly coupled to the experimental potentials, as indicated by the red dots in Figure 1. Two more electrodes were cycled for 500 cycles to vacuum chamber to avoid any air exposure. Technically, we noticed the oxidized oxygen signals in its charged and discharged states with the electrochemical profile reported before [18]. All samples some battweerryeehlaencdtlreodduensd,ersapheicgihalpluyrsitoymAreeLniv-ironmbeanttebreyfomreabteeinrgiadlsir,ewctliyllcdouepclredatsoetihne einxptenrismiteyntualpon soft vacuum chamber to avoid any air exposure. Technically, we noticed the oxidized oxygen signals in X-ray radiation, which behaves the same as Li2O2 [9]. We therefore carefully tested the radiation some battery electrodes, especially some Li-ion battery materials, will decrease in intensity upon soft dependence of several systems, which will be reported separately. Nonetheless, the radiation sensitivity X-ray radiation, which behaves the same as Li2O2 [9]. We therefore carefully tested the radiation leads to only underestimated oxidized oxygen signals in sensitive materials, which does not affect dependence of several systems, which will be reported separately. Nonetheless, the radiation the qualitative detection of the emergence of oxygen redox. However, extra caution is necessary for sensitivity leads to only underestimated oxidized oxygen signals in sensitive materials, which does quantitatinvoetafnfeacltytshies,qwuahlitcahtivisefdeeatescitbiolentohfrtohuegehmaercgoencterolfleodxyXge-nraryedooxs.eHaonwdevmera,ienxtraaincianugtioannisitinerant necessary for quantitative analysis, which is feasible through a controlled X-ray dose and sample during experiments. maintaining an itinerant sample during experiments. Figure 1. (a) Electrochemical profile and crystal structure of LR-NMC material. The crystal structure Figure 1. (a) Electrochemical profile and crystal structure of LR-NMC material. The crystal structure oftheLR-oNftMheCLRm-NaMteCriamlabterlioalnbgeslotnogsthtoetChe2/C2m/mspspaaccegroupp..TThehheighhigvholtvaogletapglaetepauladtueraiungdtuherifnirgstthefirst chargingcchyacrgleingcocryrcelespcornredsspotnodastloaraglearagmeaomuonutnotfofooxxygenoxxididataiotino.nS.ixSriexprreesepnrteastievnetsaatmivpelesaamtplesat different electrochemical potentials are marked as the red dots on the electrochemical profile. These different electrochemical potentials are marked as the red dots on the electrochemical profile. These electrodes, together with the charged and discharged electrodes after 500 cycles, are selected for electrodes, together with the charged and discharged electrodes after 500 cycles, are selected for mRIXS mRIXS characterizations. (b) Schematic illustration of the mRIXS system. The experimental set-up, characterizations. (b) Schematic illustration of the mRIXS system. The experimental set-up, other than other than the light source, consists mainly of a refocus mirror, spectrometer optics with a mirror and the light source, consists mainly of a refocus mirror, spectrometer optics with a mirror and a grating, a grating, and an X-ray photon detector. Reproduced from Ref. 15 with permission. and an X-ray photon detector. Reproduced from Ref. 15 with permission. mRIXS of this series of LR-NMC electrodes at different electrochemical states were collected in the ultra-high efficient iRIXS endstation at Beamline 8.0.1 of Advanced Light Source [15]. Figure 1b is mRIXS of this series of LR-NMC electrodes at different electrochemical states were collected in the ultra-high efficient iRIXS endstation at Beamline 8.0.1 of Advanced Light Source [15]. Figure 1b is the schematic illustration of the mRIXS system in the endstation, which has been detailed in previousPDF Image | Oxygen Redox Reactions in Batteries Resonant Inelastic X-ray Scattering
PDF Search Title:
Oxygen Redox Reactions in Batteries Resonant Inelastic X-ray ScatteringOriginal File Name Searched:
condensedmatter-04-00005.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |