
PDF Publication Title:
Text from PDF Page: 005
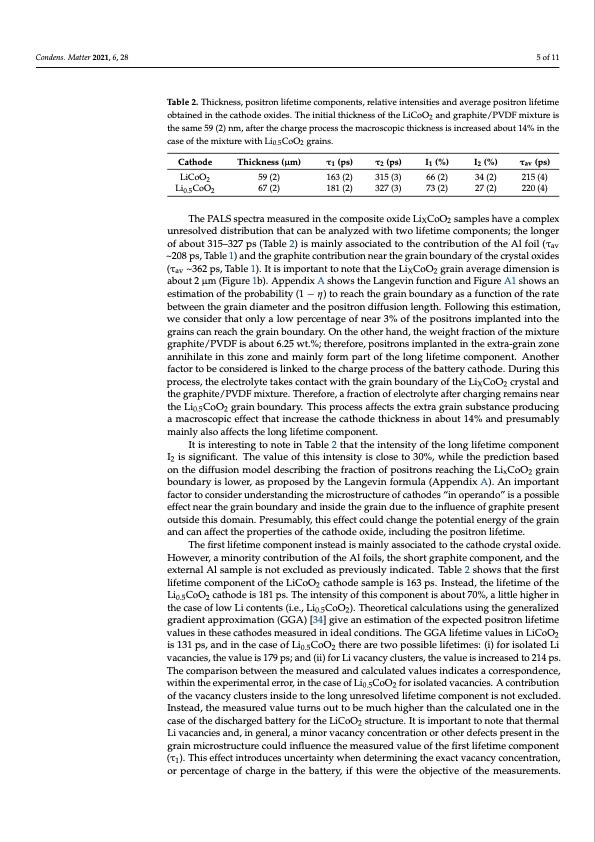
Condens. Matter 2021, 6, 28 5 of 11 Table 2. Thickness, positron lifetime components, relative intensities and average positron lifetime obtained in the cathode oxides. The initial thickness of the LiCoO2 and graphite/PVDF mixture is the same 59 (2) nm, after the charge process the macroscopic thickness is increased about 14% in the case of the mixture with Li0.5CoO2 grains. Cathode LiCoO2 Li0.5CoO2 Thickness (μm) 59 (2) 67 (2) τ1 (ps) 163 (2) 181 (2) τ2 (ps) 315 (3) 327 (3) I1 (%) 66 (2) 73 (2) I2 (%) 34 (2) 27 (2) τav (ps) 215 (4) 220 (4) The PALS spectra measured in the composite oxide LiXCoO2 samples have a complex unresolved distribution that can be analyzed with two lifetime components; the longer of about 315–327 ps (Table 2) is mainly associated to the contribution of the Al foil (τav ~208 ps, Table 1) and the graphite contribution near the grain boundary of the crystal oxides (τav ~362 ps, Table 1). It is important to note that the LiXCoO2 grain average dimension is about 2 μm (Figure 1b). Appendix A shows the Langevin function and Figure A1 shows an estimation of the probability (1 − η) to reach the grain boundary as a function of the rate between the grain diameter and the positron diffusion length. Following this estimation, we consider that only a low percentage of near 3% of the positrons implanted into the grains can reach the grain boundary. On the other hand, the weight fraction of the mixture graphite/PVDF is about 6.25 wt.%; therefore, positrons implanted in the extra-grain zone annihilate in this zone and mainly form part of the long lifetime component. Another factor to be considered is linked to the charge process of the battery cathode. During this process, the electrolyte takes contact with the grain boundary of the LiXCoO2 crystal and the graphite/PVDF mixture. Therefore, a fraction of electrolyte after charging remains near the Li0.5CoO2 grain boundary. This process affects the extra grain substance producing a macroscopic effect that increase the cathode thickness in about 14% and presumably mainly also affects the long lifetime component. It is interesting to note in Table 2 that the intensity of the long lifetime component I2 is significant. The value of this intensity is close to 30%, while the prediction based on the diffusion model describing the fraction of positrons reaching the LixCoO2 grain boundary is lower, as proposed by the Langevin formula (Appendix A). An important factor to consider understanding the microstructure of cathodes “in operando” is a possible effect near the grain boundary and inside the grain due to the influence of graphite present outside this domain. Presumably, this effect could change the potential energy of the grain and can affect the properties of the cathode oxide, including the positron lifetime. The first lifetime component instead is mainly associated to the cathode crystal oxide. However, a minority contribution of the Al foils, the short graphite component, and the external Al sample is not excluded as previously indicated. Table 2 shows that the first lifetime component of the LiCoO2 cathode sample is 163 ps. Instead, the lifetime of the Li0.5CoO2 cathode is 181 ps. The intensity of this component is about 70%, a little higher in the case of low Li contents (i.e., Li0.5CoO2). Theoretical calculations using the generalized gradient approximation (GGA) [34] give an estimation of the expected positron lifetime values in these cathodes measured in ideal conditions. The GGA lifetime values in LiCoO2 is 131 ps, and in the case of Li0.5CoO2 there are two possible lifetimes: (i) for isolated Li vacancies, the value is 179 ps; and (ii) for Li vacancy clusters, the value is increased to 214 ps. The comparison between the measured and calculated values indicates a correspondence, within the experimental error, in the case of Li0.5CoO2 for isolated vacancies. A contribution of the vacancy clusters inside to the long unresolved lifetime component is not excluded. Instead, the measured value turns out to be much higher than the calculated one in the case of the discharged battery for the LiCoO2 structure. It is important to note that thermal Li vacancies and, in general, a minor vacancy concentration or other defects present in the grain microstructure could influence the measured value of the first lifetime component (τ1). This effect introduces uncertainty when determining the exact vacancy concentration, or percentage of charge in the battery, if this were the objective of the measurements.PDF Image | Positron Annihilation Spectroscopy LiCoO2 Cathode of Lithium-Ion Batteries
PDF Search Title:
Positron Annihilation Spectroscopy LiCoO2 Cathode of Lithium-Ion BatteriesOriginal File Name Searched:
condensedmatter-06-00028.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |