
PDF Publication Title:
Text from PDF Page: 007
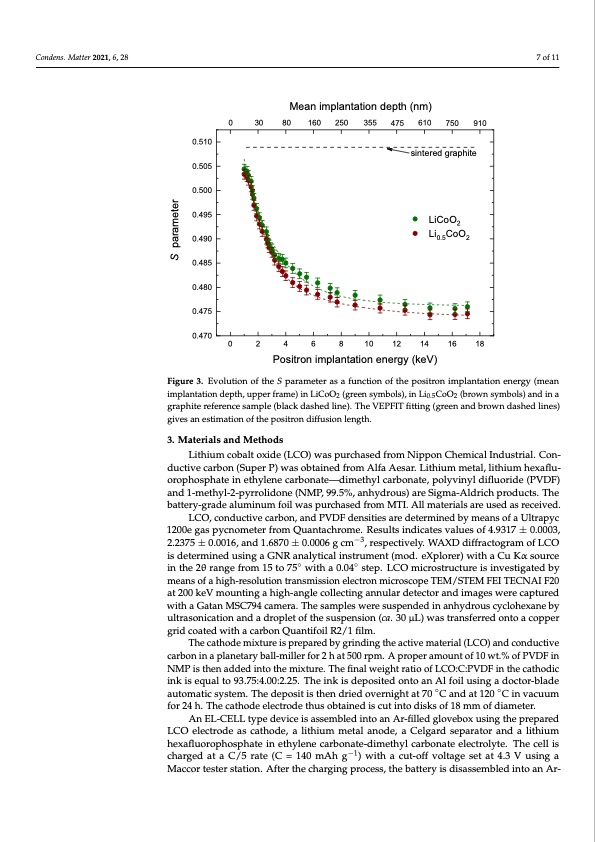
Condens. Matter 2021, 6, x FOR PEER REVIEW 7 of 12 Condens. Matter 2021, 6, 28 rationalized by a strong distortion of the positron wave-function induced by graphite re- ported by Cartier et al. [44]. In fact, if the positron wave-function spills over the grains it becomes less sensitive to the Li ion vacancies. Therefore, in this case, X-ray Compton and the Doppler broadening experiments can give similar results. Mean implantation depth (nm) 0 30 80 160 250 355 475 610 750 910 0.510 0.505 0.500 0.495 0.490 0.485 0.480 0.475 0.470 Figure 3. Evolution of the S parameter as a function of the positron implantation energy (mean Figure 3. Evolution of the S parameter as a function of the positron implantation energy (mean 0 2 4 6 8 10 12 14 16 18 Positron implantation energy (keV) implantation depth, upper frame) in LiCoO (green symbols), in Li CoO (brown symbols) and in a 2 0.52 implantation depth, upper frame) in LiCoO2 (green symbols), in Li0.5CoO2 (brown symbols) and in graphite reference sample (black dashed line). The VEPFIT fitting (green and brown dashed lines) a graphite reference sample (black dashed line). The VEPFIT fitting (green and brown dashed lines) givgeisveasnaensteimstiamtiaotnioonf othfethpeopsiotrsoitnrodnifdfuifsfuiosniolnenlegnthg.th. 7 of 11 sintered graphite LiCoO2 Li0.5CoO2 3. Materials and Methods Figure 3 shows a best-fit procedure for the experimental data (green and brown Lithium cobalt oxide (LCO) was purchased from Nippon Chemical Industrial. Con- dashed lines) called VEPFIT [45] based on the solution of the diffusion equation for posi- ductive carbon (Super P) was obtained from Alfa Aesar. Lithium metal, lithium hexaflu- trons in layers, considering the energy-dependent positron implantation profiles (Makhov orophosphate in ethylene carbonate—dimethyl carbonate, polyvinyl difluoride (PVDF) profiles). To fit the experimental data, a one-layer model was used, comprising the sample and 1-methyl-2-pyrrolidone (NMP, 99.5%, anhydrous) are Sigma-Aldrich products. The surface and the bulk. It was possible to estimate a set of parameters as the positron diffu- battery-grade aluminum foil was purchased from MTI. All materials are used as received. sion length and S parameter of the surface and bulk knowing the film mass density (Table LCO, conductive carbon, and PVDF densities are determined by means of a Ultrapyc 3). The positron diffusion length L+ is about 60 nm, and it tends to be shorter in the case of 1200e gas pycnometer from Quantachrome. Results indicates values of 4.9317 ± 0.0003, Li0.5CoO2, but it does not differ much within experimental error. 2.2375 ± 0.0016, and 1.6870 ± 0.0006 g cm−3, respectively. WAXD diffractogram of LCO is determined using a GNR analytical instrument (mod. eXplorer) with a Cu Kα source Table 3. Positron diffusion length and S parameter of the surface and bulk. in the 2θ range from 15 to 75◦ with a 0.04◦ step. LCO microstructure is investigated by Cathode L+ (nm) Ssurface Sbulk means of a high-resolution transmission electron microscope TEM/STEM FEI TECNAI F20 LiCoO2 60 (3) 0.506 (1) 0.4760 (7) at 200 keV mounting a high-angle collecting annular detector and images were captured wiLthi0.a5CGoaOta2nMSC794came5ra5.(T3)hesamplesweresu0s.p5e0n5d(1ed)inanhydrou0s.4c7y4c2lo(h7)exaneby ultrasonication and a droplet of the suspension (ca. 30 μL) was transferred onto a copper 3. MgriadtecroialtsedanwditMh aetchaordbosn Quantifoil R2/1 film. The cathode mixture is prepared by grinding the active material (LCO) and conductive Lithium cobalt oxide (LCO) was purchased from Nippon Chemical Industrial. Con- carbon in a planetary ball-miller for 2 h at 500 rpm. A proper amount of 10 wt.% of PVDF in ductive carbon (Super P) was obtained from Alfa Aesar. Lithium metal, lithium hexafluor- NMP is then added into the mixture. The final weight ratio of LCO:C:PVDF in the cathodic ophosphate in ethylene carbonate—dimethyl carbonate, polyvinyl difluoride (PVDF) and ink is equal to 93.75:4.00:2.25. The ink is deposited onto an Al foil using a doctor-blade 1-methyl-2-pyrrolidone (NMP, 99.5%, anhydrous) are Sigma-Aldrich products. The bat- automatic system. The deposit is then dried overnight at 70 ◦C and at 120 ◦C in vacuum tery-grade aluminum foil was purchased from MTI. All materials are used as received. for 24 h. The cathode electrode thus obtained is cut into disks of 18 mm of diameter. An EL-CELL type device is assembled into an Ar-filled glovebox using the prepared LCO electrode as cathode, a lithium metal anode, a Celgard separator and a lithium hexafluorophosphate in ethylene carbonate-dimethyl carbonate electrolyte. The cell is charged at a C/5 rate (C = 140 mAh g−1) with a cut-off voltage set at 4.3 V using a Maccor tester station. After the charging process, the battery is disassembled into an Ar- S parameterPDF Image | Positron Annihilation Spectroscopy LiCoO2 Cathode of Lithium-Ion Batteries
PDF Search Title:
Positron Annihilation Spectroscopy LiCoO2 Cathode of Lithium-Ion BatteriesOriginal File Name Searched:
condensedmatter-06-00028.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |