
PDF Publication Title:
Text from PDF Page: 004
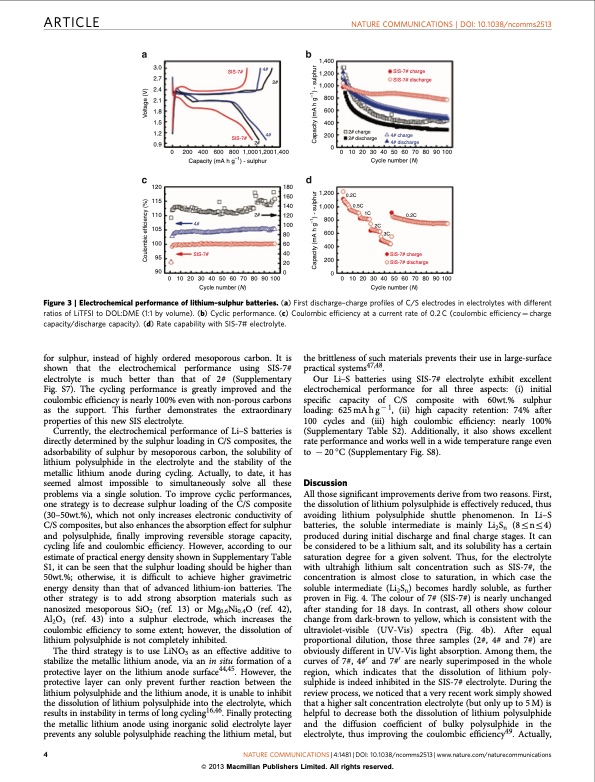
ARTICLE NATURE COMMUNICATIONS | DOI: 10.1038/ncomms2513 3.0 2.7 2.4 2.1 1.8 1.5 1.2 0.9 120 115 110 105 100 95 90 SIS-7# SIS-7# 4# 4# 2# 1,400 1,200 1,000 800 600 400 200 0 1,200 1,000 800 600 400 SIS-7# charge SIS-7# discharge 4# charge 4# discharge 2# 0 200 400 600 800 1,0001,2001,400 2# charge 2# discharge Capacity (mA h g–1) - sulphur 0 10 20 30 40 50 60 70 80 90 100 Cycle number (N) 180 160 140 120 100 80 60 0.2C 0.5C 4# SIS-7# 2C 0 102030405060708090100 Cycle number (N) 0 102030405060708090100 Cycle number (N) 2# 1C 0.2C 40 20 200 00 SIS-7# charge SIS-7# discharge Figure 3 | Electrochemical performance of lithium–sulphur batteries. (a) First discharge–charge profiles of C/S electrodes in electrolytes with different ratios of LiTFSI to DOL:DME (1:1 by volume). (b) Cyclic performance. (c) Coulombic efficiency at a current rate of 0.2 C (coulombic efficiency 1⁄4 charge capacity/discharge capacity). (d) Rate capability with SIS-7# electrolyte. for sulphur, instead of highly ordered mesoporous carbon. It is shown that the electrochemical performance using SIS-7# electrolyte is much better than that of 2# (Supplementary Fig. S7). The cycling performance is greatly improved and the coulombic efficiency is nearly 100% even with non-porous carbons as the support. This further demonstrates the extraordinary properties of this new SIS electrolyte. Currently, the electrochemical performance of Li–S batteries is directly determined by the sulphur loading in C/S composites, the adsorbability of sulphur by mesoporous carbon, the solubility of lithium polysulphide in the electrolyte and the stability of the metallic lithium anode during cycling. Actually, to date, it has seemed almost impossible to simultaneously solve all these problems via a single solution. To improve cyclic performances, one strategy is to decrease sulphur loading of the C/S composite (30–50wt.%), which not only increases electronic conductivity of C/S composites, but also enhances the absorption effect for sulphur and polysulphide, finally improving reversible storage capacity, cycling life and coulombic efficiency. However, according to our estimate of practical energy density shown in Supplementary Table S1, it can be seen that the sulphur loading should be higher than 50wt.%; otherwise, it is difficult to achieve higher gravimetric energy density than that of advanced lithium-ion batteries. The other strategy is to add strong absorption materials such as nanosized mesoporous SiO2 (ref. 13) or Mg0.6Ni0.4O (ref. 42), Al2O3 (ref. 43) into a sulphur electrode, which increases the coulombic efficiency to some extent; however, the dissolution of lithium polysulphide is not completely inhibited. The third strategy is to use LiNO3 as an effective additive to stabilize the metallic lithium anode, via an in situ formation of a protective layer on the lithium anode surface44,45. However, the protective layer can only prevent further reaction between the lithium polysulphide and the lithium anode, it is unable to inhibit the dissolution of lithium polysulphide into the electrolyte, which results in instability in terms of long cycling16,46. Finally protecting the metallic lithium anode using inorganic solid electrolyte layer prevents any soluble polysulphide reaching the lithium metal, but the brittleness of such materials prevents their use in large-surface practical systems47,48. Our Li–S batteries using SIS-7# electrolyte exhibit excellent electrochemical performance for all three aspects: (i) initial specific capacity of C/S composite with 60wt.% sulphur loading: 625 mA h g 1, (ii) high capacity retention: 74% after 100 cycles and (iii) high coulombic efficiency: nearly 100% (Supplementary Table S2). Additionally, it also shows excellent rate performance and works well in a wide temperature range even to 20 °C (Supplementary Fig. S8). Discussion All those significant improvements derive from two reasons. First, the dissolution of lithium polysulphide is effectively reduced, thus avoiding lithium polysulphide shuttle phenomenon. In Li–S batteries, the soluble intermediate is mainly Li2Sn (8rnr4) produced during initial discharge and final charge stages. It can be considered to be a lithium salt, and its solubility has a certain saturation degree for a given solvent. Thus, for the electrolyte with ultrahigh lithium salt concentration such as SIS-7#, the concentration is almost close to saturation, in which case the soluble intermediate (Li2Sn) becomes hardly soluble, as further proven in Fig. 4. The colour of 7# (SIS-7#) is nearly unchanged after standing for 18 days. In contrast, all others show colour change from dark-brown to yellow, which is consistent with the ultraviolet-visible (UV-Vis) spectra (Fig. 4b). After equal proportional dilution, those three samples (2#, 4# and 7#) are obviously different in UV-Vis light absorption. Among them, the curves of 7#, 4#0 and 7#0 are nearly superimposed in the whole region, which indicates that the dissolution of lithium poly- sulphide is indeed inhibited in the SIS-7# electrolyte. During the review process, we noticed that a very recent work simply showed that a higher salt concentration electrolyte (but only up to 5 M) is helpful to decrease both the dissolution of lithium polysulphide and the diffusion coefficient of bulky polysulphide in the electrolyte, thus improving the coulombic efficiency49. Actually, 4 NATURE COMMUNICATIONS | 4:1481 | DOI: 10.1038/ncomms2513 | www.nature.com/naturecommunications & 2013 Macmillan Publishers Limited. All rights reserved. 3C Coulombic efficiency (%) Voltage (V) Capacity (mA h g–1) - sulphur Capacity (mA h g–1) - sulphurPDF Image | Solvent-in-Salt electrolyte for high-energy rechargeable metallic lithium batteries
PDF Search Title:
Solvent-in-Salt electrolyte for high-energy rechargeable metallic lithium batteriesOriginal File Name Searched:
ncomms2513.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |