
PDF Publication Title:
Text from PDF Page: 002
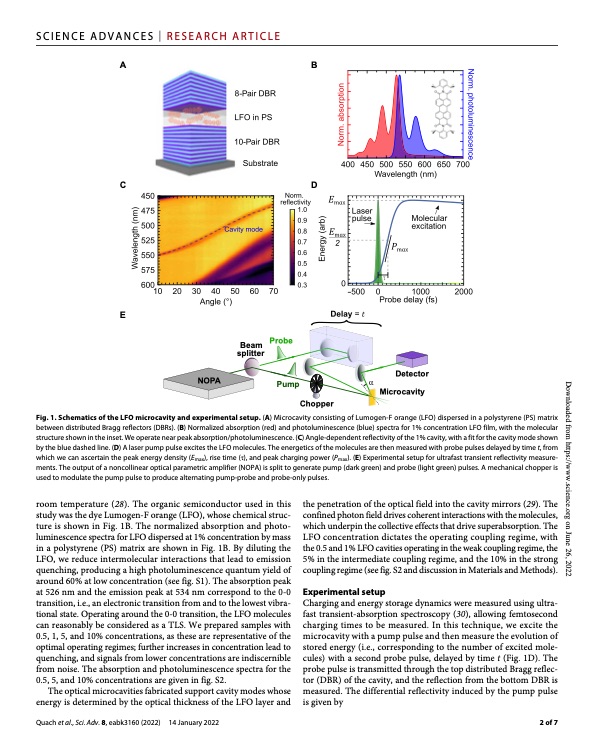
SCIENCE ADVANCES | RESEARCH ARTICLE AB CD E NOPA Pump Chopper Detector Microcavity Beam Probe splitter Fig. 1. Schematics of the LFO microcavity and experimental setup. (A) Microcavity consisting of Lumogen-F orange (LFO) dispersed in a polystyrene (PS) matrix between distributed Bragg reflectors (DBRs). (B) Normalized absorption (red) and photoluminescence (blue) spectra for 1% concentration LFO film, with the molecular structure shown in the inset. We operate near peak absorption/photoluminescence. (C) Angle-dependent reflectivity of the 1% cavity, with a fit for the cavity mode shown by the blue dashed line. (D) A laser pump pulse excites the LFO molecules. The energetics of the molecules are then measured with probe pulses delayed by time t, from which we can ascertain the peak energy density (Emax), rise time (), and peak charging power (Pmax). (E) Experimental setup for ultrafast transient reflectivity measure- ments. The output of a noncollinear optical parametric amplifier (NOPA) is split to generate pump (dark green) and probe (light green) pulses. A mechanical chopper is used to modulate the pump pulse to produce alternating pump-probe and probe-only pulses. room temperature (28). The organic semiconductor used in this study was the dye Lumogen-F orange (LFO), whose chemical struc- ture is shown in Fig. 1B. The normalized absorption and photo- luminescence spectra for LFO dispersed at 1% concentration by mass in a polystyrene (PS) matrix are shown in Fig. 1B. By diluting the LFO, we reduce intermolecular interactions that lead to emission quenching, producing a high photoluminescence quantum yield of around 60% at low concentration (see fig. S1). The absorption peak at 526 nm and the emission peak at 534 nm correspond to the 0-0 transition, i.e., an electronic transition from and to the lowest vibra- tional state. Operating around the 0-0 transition, the LFO molecules can reasonably be considered as a TLS. We prepared samples with 0.5, 1, 5, and 10% concentrations, as these are representative of the optimal operating regimes; further increases in concentration lead to quenching, and signals from lower concentrations are indiscernible from noise. The absorption and photoluminescence spectra for the 0.5, 5, and 10% concentrations are given in fig. S2. The optical microcavities fabricated support cavity modes whose energy is determined by the optical thickness of the LFO layer and Quach et al., Sci. Adv. 8, eabk3160 (2022) 14 January 2022 the penetration of the optical field into the cavity mirrors (29). The confined photon field drives coherent interactions with the molecules, which underpin the collective effects that drive superabsorption. The LFO concentration dictates the operating coupling regime, with the 0.5 and 1% LFO cavities operating in the weak coupling regime, the 5% in the intermediate coupling regime, and the 10% in the strong coupling regime (see fig. S2 and discussion in Materials and Methods). Experimental setup Charging and energy storage dynamics were measured using ultra- fast transient-absorption spectroscopy (30), allowing femtosecond charging times to be measured. In this technique, we excite the microcavity with a pump pulse and then measure the evolution of stored energy (i.e., corresponding to the number of excited mole- cules) with a second probe pulse, delayed by time t (Fig. 1D). The probe pulse is transmitted through the top distributed Bragg reflec- tor (DBR) of the cavity, and the reflection from the bottom DBR is measured. The differential reflectivity induced by the pump pulse is given by Downloaded from https://www.science.org on June 26, 2022 2 of 7PDF Image | Superabsorption organic microcavity Toward a quantum battery
PDF Search Title:
Superabsorption organic microcavity Toward a quantum batteryOriginal File Name Searched:
sciadvabk3160.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |