
PDF Publication Title:
Text from PDF Page: 007
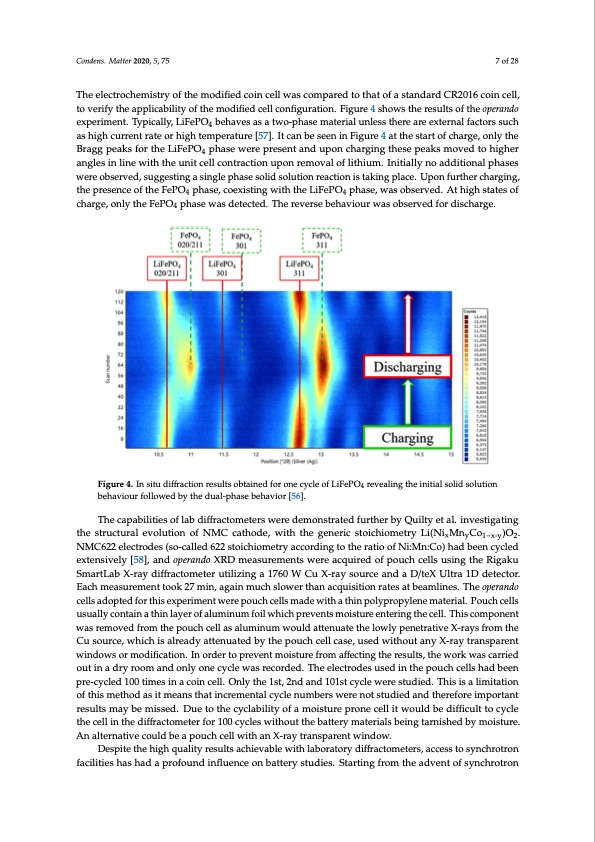
much slower than synchrotron equivalents (ca. 10 s acquisition time) showed the improvements in performing lab-based operando studies in close to real time. Patterns were obtained continuously whilst the battery was charged at a C-rate of C/10. Variables such as the C-rate can be chosen to allow for data acquisition to be performed at multiple points throughout the cycle, at a time scale appropriate for the acquisition. For example, with an acquisition time of 8 min, a C-rate of C/2 would Condens. Matter 2020, 5, 75 7 of 28 give 15 data points along the charge curve. The cell used in this study was a modified coin cell with a 10 mm Kapton window. The electrochemistry of the modified coin cell was compared to that of a TshtaenedleacrdtroCcRh2em01i6stcroyinofctehlel, mtoovdeifiriefyd cthoeinacpeplliwcaabsilcitoymopfatrheedmtootdhiafiteodf caeslltacnodnafirgduCraRti2o0n1.6Fcigouinrece4ll, toshvoewrisfyththeeraespuplltiscaobfiltihtyeofpetrhaenmdooedxifipedrimceelnlct.oTnyfipgiucraaltlyio,nL.iFiegPuOre4 b4eshhaovwesathsearetwsuol-tsphoafstehemoapteerrainadlo euxnplersismtehnetr.eTayrpeiecaxltleyr,nLailFfeaPcOtorsbseuhcahveasahsigahtwcuor-rpehnatsreamteaotrerhiaiglhuntelemspsethraetrueraer[e5e7x].teItrncalnfabcetosersensuinch 4 aFsihguigrhec4uartretnhtersattaertoorfhcighhartgeem,ponerlyattuhreB[5r7a]g.gItpceaankbsefosreethneinLiFiegPuOre44pahtastheewsetarretporfecsheanrtgaen,donulpyotnhe BcrhaagrgipnegatkhsesfoerptehaeksLimFeoPvOedtpohhaisgehwerearnegpleressiennltinaendwuithpotnhecuhnaritgicneglltchoensterapcteiaoknsumponverdemtovhaiglhofer 4 alnitghlieusmin. Iliniteiawlliythntoheauddniticoenlallcopnhtarsaecstiownerueponbsreermveodv,asluogfglietshtiungm.aInsintigalelypnhoasaeddsoitliodnasollpuhtiaosnes reaction is taking place. Upon further charging, the presence of the FePO4 phase, coexisting with the were observed, suggesting a single phase solid solution reaction is taking place. Upon further charging, LiFePO4 phase, was observed. At high states of charge, only the FePO4 phase was detected. The the presence of the FePO4 phase, coexisting with the LiFePO4 phase, was observed. At high states of reverse behaviour was observed for discharge. charge, only the FePO4 phase was detected. The reverse behaviour was observed for discharge. Figure 4. In situ diffraction results obtained for one cycle of LiFePO4 revealing the initial solid solution Figure 4. In situ diffraction results obtained for one cycle of LiFePO4 revealing the initial solid solution behaviour followed by the dual-phase behavior [56]. behaviour followed by the dual-phase behavior [56]. The capabilities of lab diffractometers were demonstrated further by Quilty et al. investigating The capabilities of lab diffractometers were demonstrated further by Quilty et al. investigating the structural evolution of NMC cathode, with the generic stoichiometry Li(NixMnyCo1−x-y)O2. the structural evolution of NMC cathode, with the generic stoichiometry Li(NixMnyCo1−x-y)O2. NMC622 electrodes (so-called 622 stoichiometry according to the ratio of Ni:Mn:Co) had been cycled NMC622 electrodes (so-called 622 stoichiometry according to the ratio of Ni:Mn:Co) had been cycled extensively [58], and operando XRD measurements were acquired of pouch cells using the Rigaku extensively [58], and operando XRD measurements were acquired of pouch cells using the Rigaku SmartLab X-ray diffractometer utilizing a 1760 W Cu X-ray source and a D/teX Ultra 1D detector. SmartLab X-ray diffractometer utilizing a 1760 W Cu X-ray source and a D/teX Ultra 1D detector. Each measurement took 27 min, again much slower than acquisition rates at beamlines. The operando Each measurement took 27 min, again much slower than acquisition rates at beamlines. The operando cells adopted for this experiment were pouch cells made with a thin polypropylene material. Pouch cells cells adopted for this experiment were pouch cells made with a thin polypropylene material. Pouch usually contain a thin layer of aluminum foil which prevents moisture entering the cell. This component cells usually contain a thin layer of aluminum foil which prevents moisture entering the cell. This was removed from the pouch cell as aluminum would attenuate the lowly penetrative X-rays from the Cu source, which is already attenuated by the pouch cell case, used without any X-ray transparent windows or modification. In order to prevent moisture from affecting the results, the work was carried out in a dry room and only one cycle was recorded. The electrodes used in the pouch cells had been pre-cycled 100 times in a coin cell. Only the 1st, 2nd and 101st cycle were studied. This is a limitation of this method as it means that incremental cycle numbers were not studied and therefore important results may be missed. Due to the cyclability of a moisture prone cell it would be difficult to cycle the cell in the diffractometer for 100 cycles without the battery materials being tarnished by moisture. An alternative could be a pouch cell with an X-ray transparent window. Despite the high quality results achievable with laboratory diffractometers, access to synchrotron facilities has had a profound influence on battery studies. Starting from the advent of synchrotronPDF Image | Synchrotron-Based X-ray Diffraction for Lithium-Ion Batteries
PDF Search Title:
Synchrotron-Based X-ray Diffraction for Lithium-Ion BatteriesOriginal File Name Searched:
condensedmatter-05-00075.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |