
PDF Publication Title:
Text from PDF Page: 011
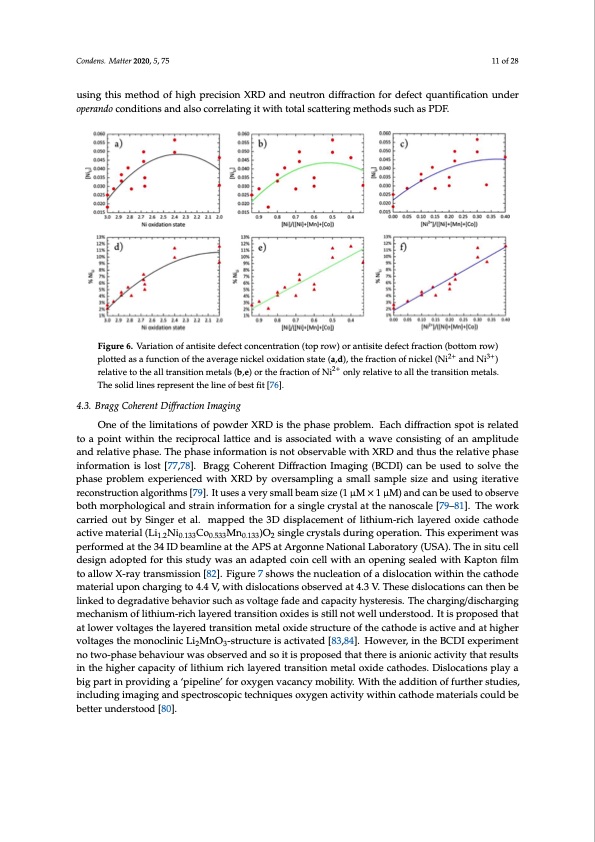
in NMC compounds. Contrary to popular belief, it was found that the defect concentration was not driven purely by the size similarity of Ni2+ and Li+ ions but rather the average size of transition-metal sites as seen in Figure 6 because the number of PAS defects are seen to scale linearly with the absolute amount of Ni2+ ions present (in Figure 6f) rather than the fraction of Ni2+ ions present (Figure 6d). This phenomena occurs because the of the larger size of Ni2+ ions compared to Ni3+ ions, pushing apart the Conodxeyngs.eMnaattteor m202s0i,n5, t7h5e layer between which the transition metals occupy octahedral sites. A be1t1teorf 28 understanding of the local structure, how it evolves during cycling and how it is affected by synthesis conditions is required to understand the degradation mechanisms of LIBs. This would be possible using this method of high precision XRD and neutron diffraction for defect quantification under using this method of high precision XRD and neutron diffraction for defect quantification under operando conditions and also correlating it with total scattering methods such as PDF. operando conditions and also correlating it with total scattering methods such as PDF. FiFgiugruer6e.6V. aVraiaritaiotinonofofanantitsiistieteddeeffeeccttcconcentration (toprow))orraanntitsisitieteddefeefcetcftrfarcatciotinon(b(obtototmtomrowro)w) 22++ 3+3+ ploptlotetdtedasaasfaufnucntciotinonofotfhteheavaevreargageennicikckeellooxxididaattiionssttatte((a,,d),,thefractionofnickel(Nii ananddNNii) ) 2++ relraetliavteivteotothtehealalltlrtarnansistitoionnmmeetatalsls(b(b,e,e))orrtthefractionofNi oonnlylyrerlealtaitvievetotoalalltlhtehteratrnasnitsioitniomnemtaeltsa.ls. ThTehseosliodlidlinlienserserperperseesnetnththeelilnineeooffbbeessttfifitt[[7766]].. 4.3. Bragg Coherent Diffraction Imaging 4.3. Bragg Coherent Diffraction Imaging OOneneofofththeelilimiittations of powddeerrXXRRDDisitshtehpehpahseasperopbrloebml.eEma.chEdacifhfradcitffiorancstpiont isproetlaitsedretloataed topaoipnotiwntitwhiinthtihnetrheecirpercoicparlolcaattliclaettaincedaisndasissocaisasteodciawteitdhwaiwthavaewcoanvseisctoingsiostfinagnoamfapnlitaumdeplaintudde anrdelraetilvaetivpehpashea.sTe.hTehpehpasheasienfionrfmoramtioantioisnnisontotbosebrsvearbvlaebwleiwthitXhRXDRDanadntdhuthsutshethreerlaetliavteivpehpahsaese inifnofromrmataiotinonisisllostt [77,78].BrBargaggCoChoehrenrtenDtifDfraiffctriaocntiIomnaIgminagg(iBnCgD(IB)CcaDnI)becaunsebdetousoeldvetothseoplvheastehe phparsoeblpermobleexmpereixepneceridenwceidthwXitRhDXRbyDobvyerosvaemrspalimngpliangsmaaslmlaslalmsapmlepsleizesizaenadndusuinsgingiteitreartaivteive reconstruction algorithms [79]. It uses a very small beam size (1 μM × 1 μM) and can be used to reconstruction algorithms [79]. It uses a very small beam size (1 μM × 1 μM) and can be used to observe observe both morphological and strain information for a single crystal at the nanoscale [79–81]. The both morphological and strain information for a single crystal at the nanoscale [79–81]. The work work carried out by Singer et al. mapped the 3D displacement of lithium-rich layered oxide cathode carried out by Singer et al. mapped the 3D displacement of lithium-rich layered oxide cathode active material (Li1.2Ni0.133Co0.533Mn0.133)O2 single crystals during operation. This experiment was active material (Li1.2Ni0.133Co0.533Mn0.133)O2 single crystals during operation. This experiment was performed at the 34 ID beamline at the APS at Argonne National Laboratory (USA). The in situ cell performed at the 34 ID beamline at the APS at Argonne National Laboratory (USA). The in situ cell design adopted for this study was an adapted coin cell with an opening sealed with Kapton film to design adopted for this study was an adapted coin cell with an opening sealed with Kapton film allow X-ray transmission [82]. Figure 7 shows the nucleation of a dislocation within the cathode to allow X-ray transmission [82]. Figure 7 shows the nucleation of a dislocation within the cathode material upon charging to 4.4 V, with dislocations observed at 4.3 V. These dislocations can then be material upon charging to 4.4 V, with dislocations observed at 4.3 V. These dislocations can then be linked to degradative behavior such as voltage fade and capacity hysteresis. The charging/discharging mechanism of lithium-rich layered transition oxides is still not well understood. It is proposed that at lower voltages the layered transition metal oxide structure of the cathode is active and at higher voltages the monoclinic Li2MnO3-structure is activated [83,84]. However, in the BCDI experiment no two-phase behaviour was observed and so it is proposed that there is anionic activity that results in the higher capacity of lithium rich layered transition metal oxide cathodes. Dislocations play a big part in providing a ‘pipeline’ for oxygen vacancy mobility. With the addition of further studies, including imaging and spectroscopic techniques oxygen activity within cathode materials could be better understood [80].PDF Image | Synchrotron-Based X-ray Diffraction for Lithium-Ion Batteries
PDF Search Title:
Synchrotron-Based X-ray Diffraction for Lithium-Ion BatteriesOriginal File Name Searched:
condensedmatter-05-00075.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |