
PDF Publication Title:
Text from PDF Page: 002
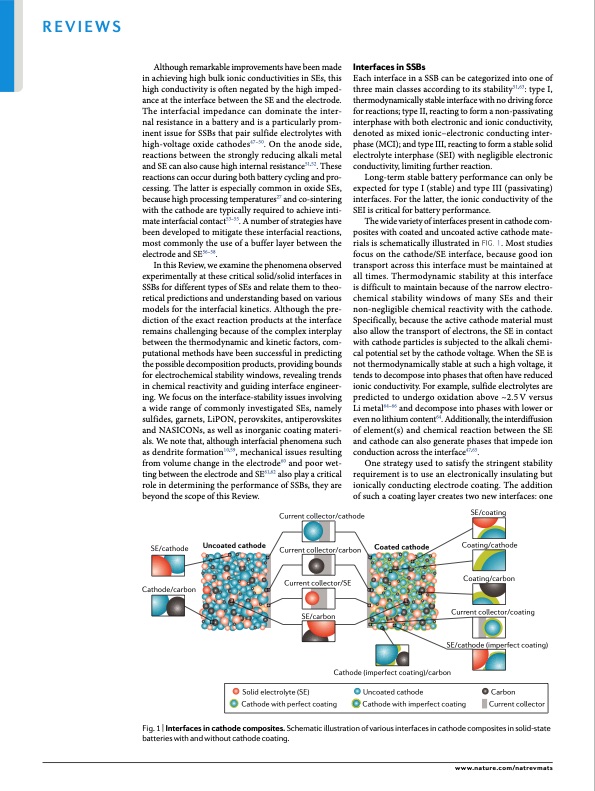
Reviews Although remarkable improvements have been made in achieving high bulk ionic conductivities in SEs, this high conductivity is often negated by the high imped- ance at the interface between the SE and the electrode. The interfacial impedance can dominate the inter- nal resistance in a battery and is a particularly prom- inent issue for SSBs that pair sulfide electrolytes with high-voltage oxide cathodes47–50. On the anode side, reactions between the strongly reducing alkali metal and SE can also cause high internal resistance51,52. These reactions can occur during both battery cycling and pro- cessing. The latter is especially common in oxide SEs, because high processing temperatures27 and co-sintering with the cathode are typically required to achieve inti- mate interfacial contact53–55. A number of strategies have been developed to mitigate these interfacial reactions, most commonly the use of a buffer layer between the electrode and SE56–58. In this Review, we examine the phenomena observed experimentally at these critical solid/solid interfaces in SSBs for different types of SEs and relate them to theo- retical predictions and understanding based on various models for the interfacial kinetics. Although the pre- diction of the exact reaction products at the interface remains challenging because of the complex interplay between the thermodynamic and kinetic factors, com- putational methods have been successful in predicting the possible decomposition products, providing bounds for electrochemical stability windows, revealing trends in chemical reactivity and guiding interface engineer- ing. We focus on the interface-stability issues involving a wide range of commonly investigated SEs, namely sulfides, garnets, LiPON, perovskites, antiperovskites and NASICONs, as well as inorganic coating materi- als. We note that, although interfacial phenomena such as dendrite formation10,59, mechanical issues resulting from volume change in the electrode60 and poor wet- ting between the electrode and SE61,62 also play a critical role in determining the performance of SSBs, they are beyond the scope of this Review. Interfaces in SSBs Each interface in a SSB can be categorized into one of three main classes according to its stability51,63: type I, thermodynamically stable interface with no driving force for reactions; type II, reacting to form a non-passivating interphase with both electronic and ionic conductivity, denoted as mixed ionic–electronic conducting inter- phase (MCI); and type III, reacting to form a stable solid electrolyte interphase (SEI) with negligible electronic conductivity, limiting further reaction. Long-term stable battery performance can only be expected for type I (stable) and type III (passivating) interfaces. For the latter, the ionic conductivity of the SEI is critical for battery performance. The wide variety of interfaces present in cathode com- posites with coated and uncoated active cathode mate- rials is schematically illustrated in fig. 1. Most studies focus on the cathode/SE interface, because good ion transport across this interface must be maintained at all times. Thermodynamic stability at this interface is difficult to maintain because of the narrow electro- chemical stability windows of many SEs and their non-negligible chemical reactivity with the cathode. Specifically, because the active cathode material must also allow the transport of electrons, the SE in contact with cathode particles is subjected to the alkali chemi- cal potential set by the cathode voltage. When the SE is not thermodynamically stable at such a high voltage, it tends to decompose into phases that often have reduced ionic conductivity. For example, sulfide electrolytes are predicted to undergo oxidation above ~2.5 V versus Li metal64–66 and decompose into phases with lower or even no lithium content64. Additionally, the interdiffusion of element(s) and chemical reaction between the SE and cathode can also generate phases that impede ion conduction across the interface47,65. One strategy used to satisfy the stringent stability requirement is to use an electronically insulating but ionically conducting electrode coating. The addition of such a coating layer creates two new interfaces: one SE/cathode Cathode/carbon Uncoated cathode Current collector/cathode Current collector/carbon Current collector/SE SE/carbon Coated cathode SE/coating Coating/cathode Coating/carbon Current collector/coating SE/cathode (imperfect coating) Cathode (imperfect coating)/carbon Solid electrolyte (SE) Uncoated cathode Carbon Cathode with perfect coating Cathode with imperfect coating Current collector Fig. 1 | interfaces in cathode composites. Schematic illustration of various interfaces in cathode composites in solid-state batteries with and without cathode coating. www.nature.com/natrevmatsPDF Image | Understanding interface stability in solid-state batteries
PDF Search Title:
Understanding interface stability in solid-state batteriesOriginal File Name Searched:
2019_xiao_nature_review.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |