
PDF Publication Title:
Text from PDF Page: 009
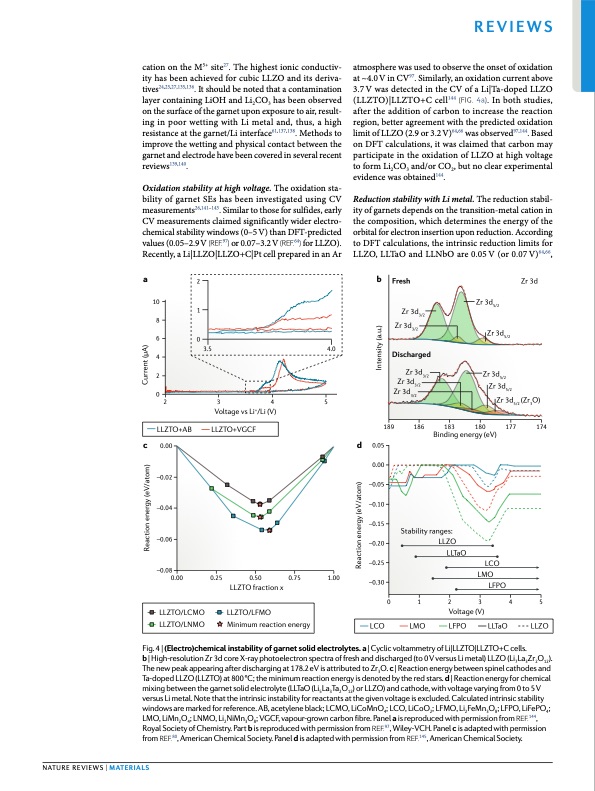
cation on the M5+ site27. The highest ionic conductiv- ity has been achieved for cubic LLZO and its deriva- tives24,25,27,135,136. It should be noted that a contamination layer containing LiOH and Li2CO3 has been observed on the surface of the garnet upon exposure to air, result- ing in poor wetting with Li metal and, thus, a high resistance at the garnet/Li interface61,137,138. Methods to improve the wetting and physical contact between the garnet and electrode have been covered in several recent reviews139,140. Oxidation stability at high voltage. The oxidation sta- bility of garnet SEs has been investigated using CV measurements26,141–143. Similar to those for sulfides, early CV measurements claimed significantly wider electro- chemical stability windows (0–5 V) than DFT-predicted values (0.05–2.9 V (ref.97) or 0.07–3.2 V (ref.64) for LLZO). Recently, a Li|LLZO|LLZO+C|Pt cell prepared in an Ar atmosphere was used to observe the onset of oxidation at ~4.0 V in CV97. Similarly, an oxidation current above 3.7 V was detected in the CV of a Li|Ta-doped LLZO (LLZTO)|LLZTO+C cell144 (fig. 4a). In both studies, after the addition of carbon to increase the reaction region, better agreement with the predicted oxidation limit of LLZO (2.9 or 3.2 V)64,66 was observed97,144. Based on DFT calculations, it was claimed that carbon may participate in the oxidation of LLZO at high voltage to form Li2CO3 and/or CO2, but no clear experimental evidence was obtained144. Reduction stability with Li metal. The reduction stabil- ity of garnets depends on the transition-metal cation in the composition, which determines the energy of the orbital for electron insertion upon reduction. According to DFT calculations, the intrinsic reduction limits for LLZO, LLTaO and LLNbO are 0.05 V (or 0.07 V)64,66, ab Fresh Zr 3d3/2 Zr 3d3/2 Discharged Zr 3d3/2 Zr 3d3/2 Zr 3d3/2 Zr 3d Zr 3d5/2 Zr 3d5/2 Reviews 10 8 6 4 2 0 Zr 3d5/2 Zr 3d5/2 2 1 0 3.5 4.0 Zr 3d 2345 5/23 (Zr O) Voltage vs Li+/Li (V) LLZTO+AB LLZTO+VGCF c 0.00 189 186 d 0.05 183 180 Binding energy (eV) 177 174 Stability ranges: LLZO LLTaO LCO LMO LFPO –0.02 –0.04 –0.06 –0.08 0.00 0.25 0.50 0.75 1.00 LLZTO fraction x 0.00 –0.05 –0.10 –0.15 –0.20 –0.25 –0.30 LCO Nature reviews | Materials LLZTO/LCMO LLZTO/LFMO LLZTO/LNMO Minimum reaction energy Fig. 4 | (electro)chemical instability of garnet solid electrolytes. a | Cyclic voltammetry of Li|LLZTO|LLZTO+C cells. b | High-resolution Zr 3d core X-ray photoelectron spectra of fresh and discharged (to 0 V versus Li metal) LLZO (Li7La3Zr2O12). The new peak appearing after discharging at 178.2 eV is attributed to Zr3O. c | Reaction energy between spinel cathodes and Ta-doped LLZO (LLZTO) at 800 °C; the minimum reaction energy is denoted by the red stars. d | Reaction energy for chemical mixing between the garnet solid electrolyte (LLTaO (Li5La3Ta2O12) or LLZO) and cathode, with voltage varying from 0 to 5 V versus Li metal. Note that the intrinsic instability for reactants at the given voltage is excluded. Calculated intrinsic stability windows are marked for reference. AB, acetylene black; LCMO, LiCoMnO4; LCO, LiCoO2; LFMO, Li2FeMn3O8; LFPO, LiFePO4; LMO, LiMn2O4; LNMO, Li2NiMn3O8; VGCF, vapour-grown carbon fibre. Panel a is reproduced with permission from ref.144, Royal Society of Chemistry. Part b is reproduced with permission from ref.97, Wiley-VCH. Panel c is adapted with permission from ref.80, American Chemical Society. Panel d is adapted with permission from ref.145, American Chemical Society. 012345 Voltage (V) LMO LFPO LLTaO LLZO Reaction energy (eV/atom) Current (μA) Reaction energy (eV/atom) Intensity (a.u.)PDF Image | Understanding interface stability in solid-state batteries
PDF Search Title:
Understanding interface stability in solid-state batteriesOriginal File Name Searched:
2019_xiao_nature_review.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |