
PDF Publication Title:
Text from PDF Page: 006
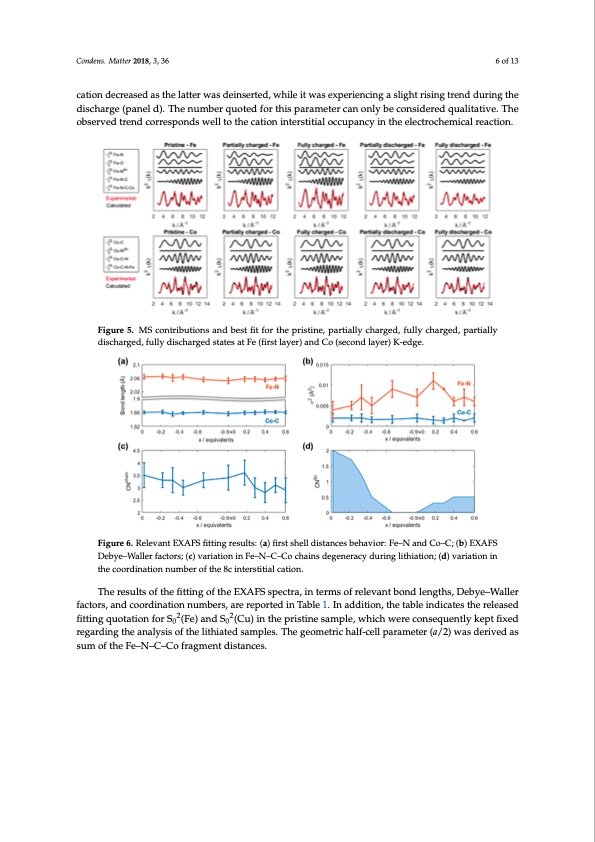
Condens. Matter 2018, 3, x FOR PEER REVIEW 6 of 13 Condens. Matter 2018, 3, x FOR PEER REVIEW 6 of 13 oscillated around a value of 3.5 during charge, while its centroid slightly decreased in the discharge. Although the large error bars set a limit to further considerations, we might assume that the oscillated around a value of 3.5 during charge, while its centroid slightly decreased in the discharge. Condens. Mliatthteirat2i0o1n8,c3o, u36ld induce a small deviation from the linearity of the orthogonal chains. Finally, the6 Foef 13 Although the large error bars set a limit to further considerations, we might assume that the coordination number to the interstitial cation decreased as the latter was deinserted, while it was lithiation could induce a small deviation from the linearity of the orthogonal chains. Finally, the Fe experiencing a slight rising trend during the discharge (panel d). The number quoted for this coordination number to the interstitial cation decreased as the latter was deinserted, while it was cation decreased as the latter was deinserted, while it was experiencing a slight rising trend during the parameter can only be considered qualitative. The observed trend corresponds well to the cation experiencing a slight rising trend during the discharge (panel d). The number quoted for this discharginet(eprsatniteialldo)c.cuTphaenncyuminbtheereqluecotrteodchfeomritchalisrepaactriaomn.etercanonlybeconsideredqualitative.The parameter can only be considered qualitative. The observed trend corresponds well to the cation observed trend corresponds well to the cation interstitial occupancy in the electrochemical reaction. interstitial occupancy in the electrochemical reaction. Figure 5. MS contributions and best fit for the pristine, partially charged, fully charged, partially Figure 5. MS contributions and best fit for the pristine, partially charged, fully charged, partially discharged, fully discharged states at Fe (first layer) and Co (second layer) K-edge. Figure 5. MS contributions and best fit for the pristine, partially charged, fully charged, partially discharged, fully discharged states at Fe (first layer) and Co (second layer) K-edge. discharged, fully discharged states at Fe (first layer) and Co (second layer) K-edge. Figure 6. Relevant EXAFS fitting results: (a) first shell distances behavior: Fe–N and Co–C; (b) EXAFS Figure 6. Relevant EXAFS fitting results: (a) first shell distances behavior: Fe–N and Co–C; (b) EXAFS Debye–Waller factors; (c) variation in Fe–N–C–Co chains degeneracy during lithiation; (d) variation in Debye–Waller factors; (c) variation in Fe–N–C–Co chains degeneracy during lithiation; (d) variation Figure 6. Relevant EXAFS fitting results: (a) first shell distances behavior: Fe–N and Co–C; (b) EXAFS the coordination number of the 8c interstitial cation. in the coordination number of the 8c interstitial cation. Debye–Waller factors; (c) variation in Fe–N–C–Co chains degeneracy during lithiation; (d) variation in the coordination number of the 8c interstitial cation. The results of the fitting of the EXAFS spectra, in terms of relevant bond lengths, Debye–Waller The results of the fitting of the EXAFS spectra, in terms of relevant bond lengths, Debye–Waller factors, and coordination numbers, are reported in Table 1. In addition, the table indicates the released factors, and coordination numbers, are reported in Table 1. In addition, the table indicates the The results of the fitting of the EXAFS spectra, in terms of relevant bond lengths, Debye–Waller fittingquotationforS2(Fe)andS2(C2u)inthep2ristinesample,whichwereconsequentlykeptfixed released fitting0quotation fo0r S0 (Fe) and S0 (Cu) in the pristine sample, which were consequently factors, and coordination numbers, are reported in Table 1. In addition, the table indicates the regardinkgetphtefiaxnedalyresgisarodfinthgetlhiethaiant2aelydssisamofptlhees2 .liTthieatgedeosmametprliecsh. aTlhf-eceglelopmaertarmicehtaelrf-(cae/ll2)pwarams deteriv(ae/d2)as released fitting quotation for S0 (Fe) and S0 (Cu) in the pristine sample, which were consequently sum of twheasFdee–rNiv–eCd–aCsosufmraogfmtheenFted–iNst–aCn–cCeso.fragment distances. kept fixed regarding the analysis of the lithiated samples. The geometric half-cell parameter (a/2) was derived as sum of the Fe–N–C–Co fragment distances.PDF Image | XAFS and XRD Study of a Prussian Blue Analogue Cathode Iron Hexacyanocobaltate
PDF Search Title:
XAFS and XRD Study of a Prussian Blue Analogue Cathode Iron HexacyanocobaltateOriginal File Name Searched:
condensedmatter-03-00036.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |