
PDF Publication Title:
Text from PDF Page: 003
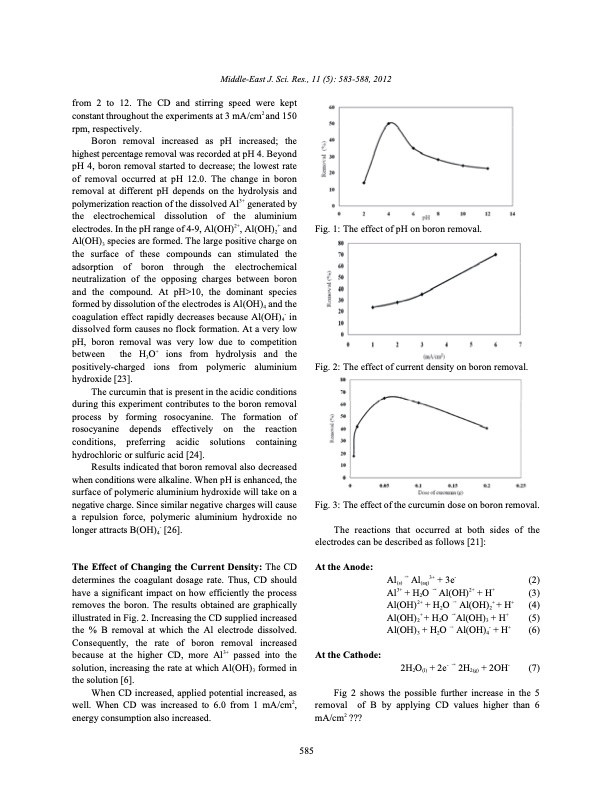
from 2 to 12. The CD and stirring speed were kept constant throughout the experiments at 3 mA/cm2 and 150 rpm, respectively. Boron removal increased as pH increased; the highest percentage removal was recorded at pH 4. Beyond pH 4, boron removal started to decrease; the lowest rate of removal occurred at pH 12.0. The change in boron removal at different pH depends on the hydrolysis and polymerization reaction of the dissolved Al3+ generated by the electrochemical dissolution of the aluminium electrodes. In the pH range of 4-9, Al(OH)2+, Al(OH)2+ and Al(OH)3 species are formed. The large positive charge on the surface of these compounds can stimulated the adsorption of boron through the electrochemical neutralization of the opposing charges between boron and the compound. At pH>10, the dominant species formed by dissolution of the electrodes is Al(OH)4 and the coagulation effect rapidly decreases because Al(OH)4- in dissolved form causes no flock formation. At a very low pH, boron removal was very low due to competition between the H3O+ ions from hydrolysis and the positively-charged ions from polymeric aluminium hydroxide [23]. The curcumin that is present in the acidic conditions during this experiment contributes to the boron removal process by forming rosocyanine. The formation of rosocyanine depends effectively on the reaction conditions, preferring acidic solutions containing hydrochloric or sulfuric acid [24]. Results indicated that boron removal also decreased when conditions were alkaline. When pH is enhanced, the surface of polymeric aluminium hydroxide will take on a negative charge. Since similar negative charges will cause a repulsion force, polymeric aluminium hydroxide no longer attracts B(OH)4- [26]. The Effect of Changing the Current Density: The CD determines the coagulant dosage rate. Thus, CD should have a significant impact on how efficiently the process removes the boron. The results obtained are graphically illustrated in Fig. 2. Increasing the CD supplied increased the % B removal at which the Al electrode dissolved. Consequently, the rate of boron removal increased because at the higher CD, more Al3+ passed into the solution, increasing the rate at which Al(OH)3 formed in the solution [6]. When CD increased, applied potential increased, as well. When CD was increased to 6.0 from 1 mA/cm2, energy consumption also increased. Fig. 1: The effect of pH on boron removal. Fig. 2: The effect of current density on boron removal. Fig. 3: The effect of the curcumin dose on boron removal. The reactions that occurred at both sides of the electrodes can be described as follows [21]: Middle-East J. Sci. Res., 11 (5): 583-588, 2012 At the Anode: At the Cathode: Al(s) Al(aq)3+ + 3e- (2) Al3+ + H2O Al(OH)2+ + H+ (3) Al(OH)2+ + H2O Al(OH)2+ + H+ (4) Al(OH)2+ + H2O Al(OH)3 + H+ (5) Al(OH)3 + H2O Al(OH)4- + H+ (6) 585 2H2O(l) + 2e- 2H2(g) + 2OH- (7) Fig 2 shows the possible further increase in the 5 removal of B by applying CD values higher than 6 mA/cm2 ???PDF Image | Boron Removal from Aqueous Solutions
PDF Search Title:
Boron Removal from Aqueous SolutionsOriginal File Name Searched:
boron-curcumin.pdfDIY PDF Search: Google It | Yahoo | Bing
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
Heat Pumps CO2 ORC Heat Pump System Platform More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |