
PDF Publication Title:
Text from PDF Page: 003
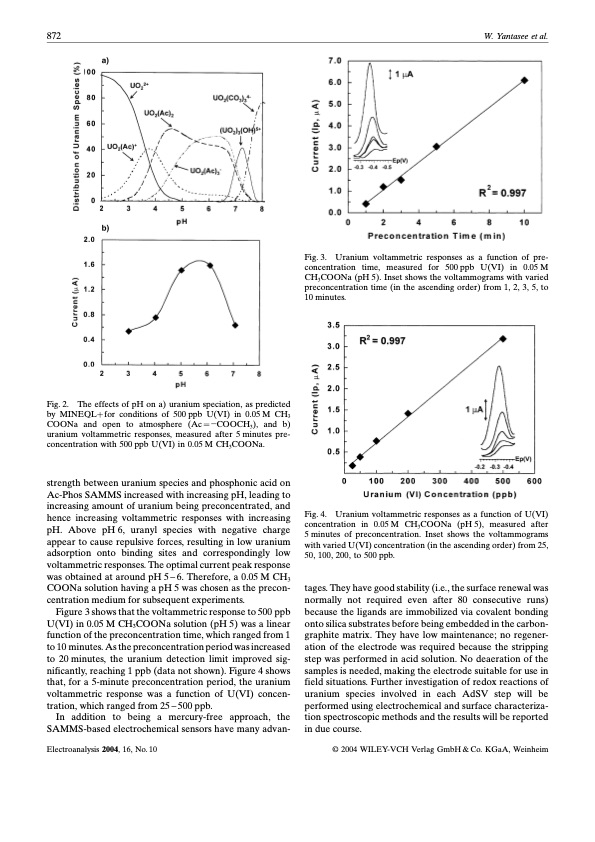
872 W. Yantasee et al. The effects of pH on a) uranium speciation, as predicted Fig. 2. by MINEQLfor conditions of 500ppb U(VI) in 0.05M CH3 COONa and open to atmosphere (Ac COOCH3), and b) uranium voltammetric responses, measured after 5 minutes pre- concentration with 500 ppb U(VI) in 0.05 M CH3COONa. strength between uranium species and phosphonic acid on Ac-Phos SAMMS increased with increasing pH, leading to increasing amount of uranium being preconcentrated, and hence increasing voltammetric responses with increasing pH. Above pH 6, uranyl species with negative charge appear to cause repulsive forces, resulting in low uranium adsorption onto binding sites and correspondingly low voltammetric responses. The optimal current peak response was obtained at around pH 5 ± 6. Therefore, a 0.05 M CH3 COONa solution having a pH 5 was chosen as the precon- centration medium for subsequent experiments. Figure 3 shows that the voltammetric response to 500 ppb U(VI) in 0.05 M CH3COONa solution (pH 5) was a linear function of the preconcentration time, which ranged from 1 to 10 minutes. As the preconcentration period was increased to 20 minutes, the uranium detection limit improved sig- nificantly, reaching 1 ppb (data not shown). Figure 4 shows that, for a 5-minute preconcentration period, the uranium voltammetric response was a function of U(VI) concen- tration, which ranged from 25 ± 500 ppb. In addition to being a mercury-free approach, the SAMMS-based electrochemical sensors have many advan- Electroanalysis 2004, 16, No. 10 Fig. 4. concentration in 0.05 M CH3COONa (pH 5), measured after 5 minutes of preconcentration. Inset shows the voltammograms with varied U(VI) concentration (in the ascending order) from 25, 50, 100, 200, to 500 ppb. tages. They have good stability (i.e., the surface renewal was normally not required even after 80 consecutive runs) because the ligands are immobilized via covalent bonding onto silica substrates before being embedded in the carbon- graphite matrix. They have low maintenance; no regener- ation of the electrode was required because the stripping step was performed in acid solution. No deaeration of the samples is needed, making the electrode suitable for use in field situations. Further investigation of redox reactions of uranium species involved in each AdSV step will be performed using electrochemical and surface characteriza- tion spectroscopic methods and the results will be reported in due course. 1 2004 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim Fig. 3. Uranium voltammetric responses as a function of pre- concentration time, measured for 500 ppb U(VI) in 0.05 M CH3COONa (pH 5). Inset shows the voltammograms with varied preconcentration time (in the ascending order) from 1, 2, 3, 5, to 10 minutes. Uranium voltammetric responses as a function of U(VI)PDF Image | Carbon Paste Electrode Modified with Carbamoylphosphonic
PDF Search Title:
Carbon Paste Electrode Modified with CarbamoylphosphonicOriginal File Name Searched:
Carbon-Paste-Electrode.pdfDIY PDF Search: Google It | Yahoo | Bing
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
Heat Pumps CO2 ORC Heat Pump System Platform More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |