
PDF Publication Title:
Text from PDF Page: 003
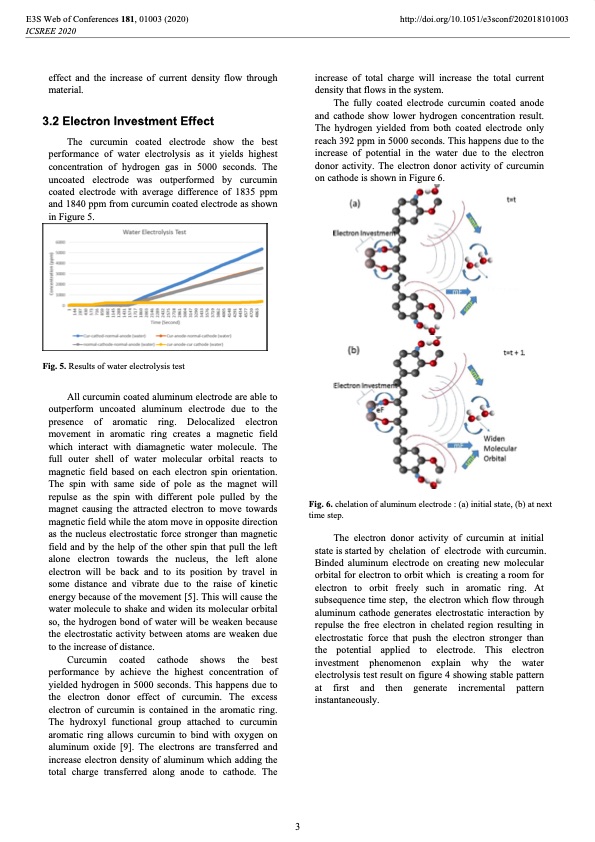
E3S Web of Conferences 181, 01003 (2020) ICSREE 2020 effect and the increase of current density flow through material. 3.2 Electron Investment Effect The curcumin coated electrode show the best performance of water electrolysis as it yields highest concentration of hydrogen gas in 5000 seconds. The uncoated electrode was outperformed by curcumin coated electrode with average difference of 1835 ppm and 1840 ppm from curcumin coated electrode as shown in Figure 5. Fig. 5. Results of water electrolysis test All curcumin coated aluminum electrode are able to outperform uncoated aluminum electrode due to the presence of aromatic ring. Delocalized electron movement in aromatic ring creates a magnetic field which interact with diamagnetic water molecule. The full outer shell of water molecular orbital reacts to magnetic field based on each electron spin orientation. The spin with same side of pole as the magnet will repulse as the spin with different pole pulled by the magnet causing the attracted electron to move towards magnetic field while the atom move in opposite direction as the nucleus electrostatic force stronger than magnetic field and by the help of the other spin that pull the left alone electron towards the nucleus, the left alone electron will be back and to its position by travel in some distance and vibrate due to the raise of kinetic energy because of the movement [5]. This will cause the water molecule to shake and widen its molecular orbital so, the hydrogen bond of water will be weaken because the electrostatic activity between atoms are weaken due to the increase of distance. Curcumin coated cathode shows the best performance by achieve the highest concentration of yielded hydrogen in 5000 seconds. This happens due to the electron donor effect of curcumin. The excess electron of curcumin is contained in the aromatic ring. The hydroxyl functional group attached to curcumin aromatic ring allows curcumin to bind with oxygen on aluminum oxide [9]. The electrons are transferred and increase electron density of aluminum which adding the total charge transferred along anode to cathode. The http://doi.org/10.1051/e3sconf/202018101003 increase of total charge will increase the total current density that flows in the system. The fully coated electrode curcumin coated anode and cathode show lower hydrogen concentration result. The hydrogen yielded from both coated electrode only reach 392 ppm in 5000 seconds. This happens due to the increase of potential in the water due to the electron donor activity. The electron donor activity of curcumin on cathode is shown in Figure 6. Fig. 6. chelation of aluminum electrode : (a) initial state, (b) at next time step. The electron donor activity of curcumin at initial state is started by chelation of electrode with curcumin. Binded aluminum electrode on creating new molecular orbital for electron to orbit which is creating a room for electron to orbit freely such in aromatic ring. At subsequence time step, the electron which flow through aluminum cathode generates electrostatic interaction by repulse the free electron in chelated region resulting in electrostatic force that push the electron stronger than the potential applied to electrode. This electron investment phenomenon explain why the water electrolysis test result on figure 4 showing stable pattern at first and then generate incremental pattern instantaneously. 3PDF Image | curcumin coated electrode on hydrogen production through water electrolysis
PDF Search Title:
curcumin coated electrode on hydrogen production through water electrolysisOriginal File Name Searched:
e3sconf_icsree2020_01003.pdfDIY PDF Search: Google It | Yahoo | Bing
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
Heat Pumps CO2 ORC Heat Pump System Platform More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |